Liquid formulation compositions, medicament delivery devices, and methods of preparation and use thereof
a technology of liquid formulation and medicament, which is applied in the direction of pharmaceutical containers, packaging foodstuffs, packaged goods, etc., can solve the problems of affecting the development of effective products, affecting the effect of drug safety, and affecting the safety of patients, etc., and achieves the effect of short tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0310]The exemplary embodiment describes a formulation and a preparation method thereof disclosed herein.
[0311]2.38 g of hydrogenated phospholipid, 0.12 g of sterol, 0.4 g of methyl paraben, 0.2 g of ethyl paraben and 0.1 g of menthol were weighed and placed in a beaker. 1 mL of anhydrous ethanol was added and dissolved with ultrasonic (40 KHz). While mixing at a high-speed stirring (6000 rpm), water was continuously added till the final volume of 100 mL, and mixed well. The formulation was filled into a containment vessel, such as sprayer or a dropping device, and the containment vessel was sealed. After the results of appearance, volume, content of main ingredient, weight per spray (or weight per drop), microbial limit and other tests were found to meet the requirements, outside packaging was performed to obtain the final product.
[0312]The surface tension of a final product was tested to be 48.851 mN / m by an automatic surface tensionmeter (US Kino, A601). The particle size distrib...
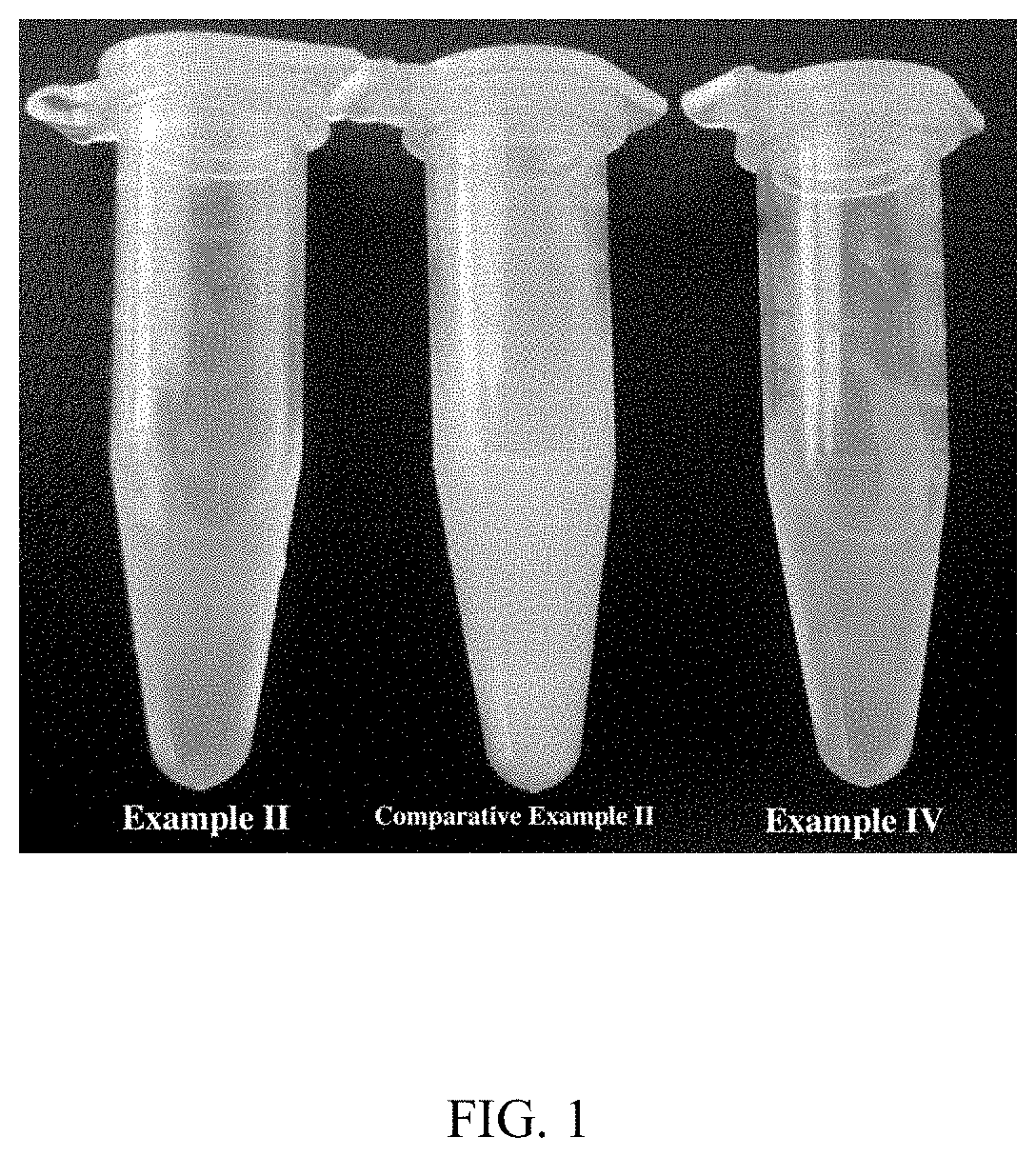
example ii
[0314]The exemplary embodiment describes a formulation and a preparation method thereof disclosed herein.
[0315]2.35 g of soya lecithin, 0.15 g of poloxamer, 0.08 g of vitamin E and 0.5 g of phenylethanol were weighed and placed in a beaker. 1 mL of anhydrous ethanol was added and dissolved with ultrasonic (40 KHz). While mixing at high speed (6000 rpm), water was continuously added till the final volume of 100 mL, and mixed well. The formulation was filled into a containment vessel, such as sprayer or a dropping device and the containment vessel was sealed. After the results of appearance, volume, content of main ingredient, weight per spray (or weight per drop), microbial limit and other tests were found to meet the requirements, outside packaging was performed for preparation to obtain the final product.
[0316]The surface tension of a final product was tested to be 16.252 mN / m by an automatic surface tensionmeter (US Kino, A601). The particle size distribution of the product was de...
example iii
[0318]The exemplary embodiment describes a formulation and a preparation method thereof disclosed herein.
[0319]3.75 g of soya lecithin and 0.64 g of vitamin E were weighed and placed in a beaker. 1 mL of anhydrous ethanol was added and dissolved with ultrasonic (40 KHz). The ethanol solution was added into 0.05 g of lauric acid monoglyceride and 99 mL of Tris-citric acid buffer solution with the pH in the range of 6.0-7.4 while a high-speed stirring (6000 rpm). The ultrasonic homogenization was further performed in an ultrasonic homogenizer. Then, the formulation was filled into containment vessel, such as an anti-contamination medicine delivery device (OSD) and sealed. After the results of appearance, volume, content of main ingredient, weight per spray (or weight per drop), microbial limit and other tests were found to meet the requirements, outside packaging was performed to obtain the final product.
[0320]The surface tension of a final product was tested to be 26.225 mN / m by an a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| zeta potential | aaaaa | aaaaa |
| volume average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap