Non-bioconvertible c3-substituted pregnenolone derivatives for use in the treatment of substance use disorders
a technology of pregnenolone and derivatives, which is applied in the field of treatment of substance use disorders, can solve the problems of not being able to achieve the full effect of treatment, further treatment opportunities are needed, and clinical results are not systematically confirmed preclinical, so as to achieve no adverse effect and reduce excessive alcohol consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
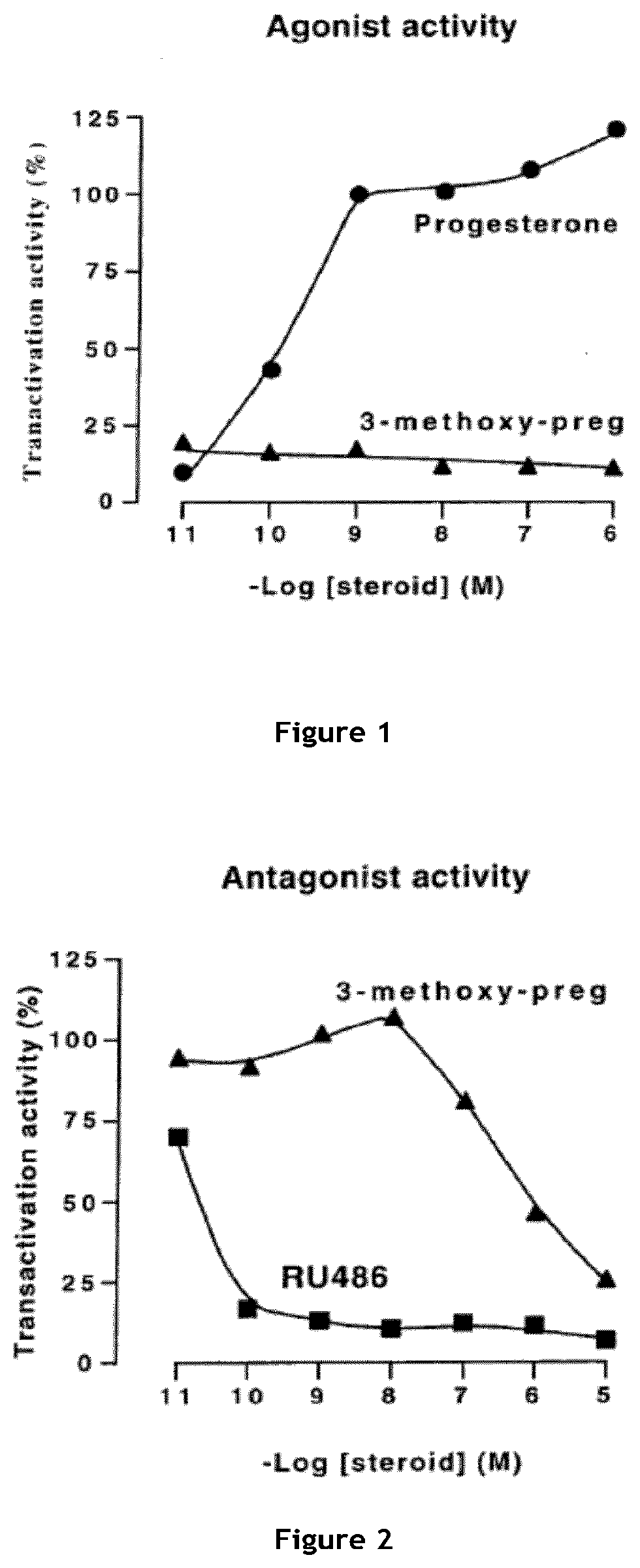
Activity of 3β-methoxy-pregnenolone on progesterone Receptor
[0155]The capacity of 3β-methoxy-pregnenolone to display progesterone activity, and thus to be considered as a progestin, was tested by assaying the activity of 3-methoxy-pregnenolone on progesterone receptor.
[0156]Indeed, progesterone is an agonist of progesterone receptor, as are all progestins. In contrast, compounds able to inhibit progesterone activity on its receptor are called progesterone receptor antagonists.
Methods
[0157]The main experimental setting used is the following: HEK293T cells were transiently transfected, using calcium phosphate precipitation technology, with expression vectors pSG5hPR (which permits expression of human progesterone receptor(PR)), pFC31-luc (contains the luciferase gene under the control of the MMTV promoter, which is in turn activated by binding of a progestin to progesterone receptor) and pcbetagal (which permits expression of betagalactosidase), and cultured during 24 hours with incre...
example 2
3β-methoxy-PREG has no Androgenic, Estrogenic, Glucocorticoid and Mineral Corticoid Activity
[0167]Binding affinity of 3β-methoxy-PREG (MAP4343) for receptors of steroid hormones was evaluated using radioligand binding assays.
[0168]MAP4343 (10 μM) was ineffective (<25% inhibition) in displacing specific radioligands from the following binding sites: Mineralocorticoid Receptor (MR), Androgen Receptor (AR), Estrogen Receptors (ERα and ERβ) and Glucorticoid Receptor (GR). The results are summarized below in Table 3 below.
TABLE 3Affinity of MAP4343 (10 μM) for steroid hormones receptors measured byradioligand binding assays. Biochemical assay results are presented as the percentinhibition of specific binding (significant responses: ≥ 50% inhibition). None of the resultsmet significance criteria at concentrations used.TargetLigandSource% inhibition*MR4.5 nM [3H] D-AldosteroneWistar Rat kidney25AR1.5 nM [3H] MiboleroneRat recombinant E. coli18ER□0.5 nM [3H] EstradiolHuman recombinant Sf9−8...
example 3
3β-methoxy-PREG has no Significant Affinity for Receptors of the Central Nervous System
[0169]MAP4343 has been screened for in vitro affinity to 80 different CNS neurotransmitters receptors using various validated binding assays.
[0170]The results show that MAP4343 has no significant affinity for any tested receptor including the ones traditionally associated with side effects or abuse liability. Results are summarized in following Table 4.
TABLE 4In vitro affinity of MAP4343 (10 μM) for CNS neurotransmitter receptorsassociated with side effects and / or abuse liability. Data are the average of twoindividual assays for each receptor and are expressed as % inhibition of the controlspecific binding of the reference compound. Results showing an inhibition higher than50% are considered to represen significant effects of the test compound. MAP4343showed no significant effects on any of the tested receptor at the concentration used.% InhibitionReceptor of controlfamilyTargetLigandSourcespecifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com