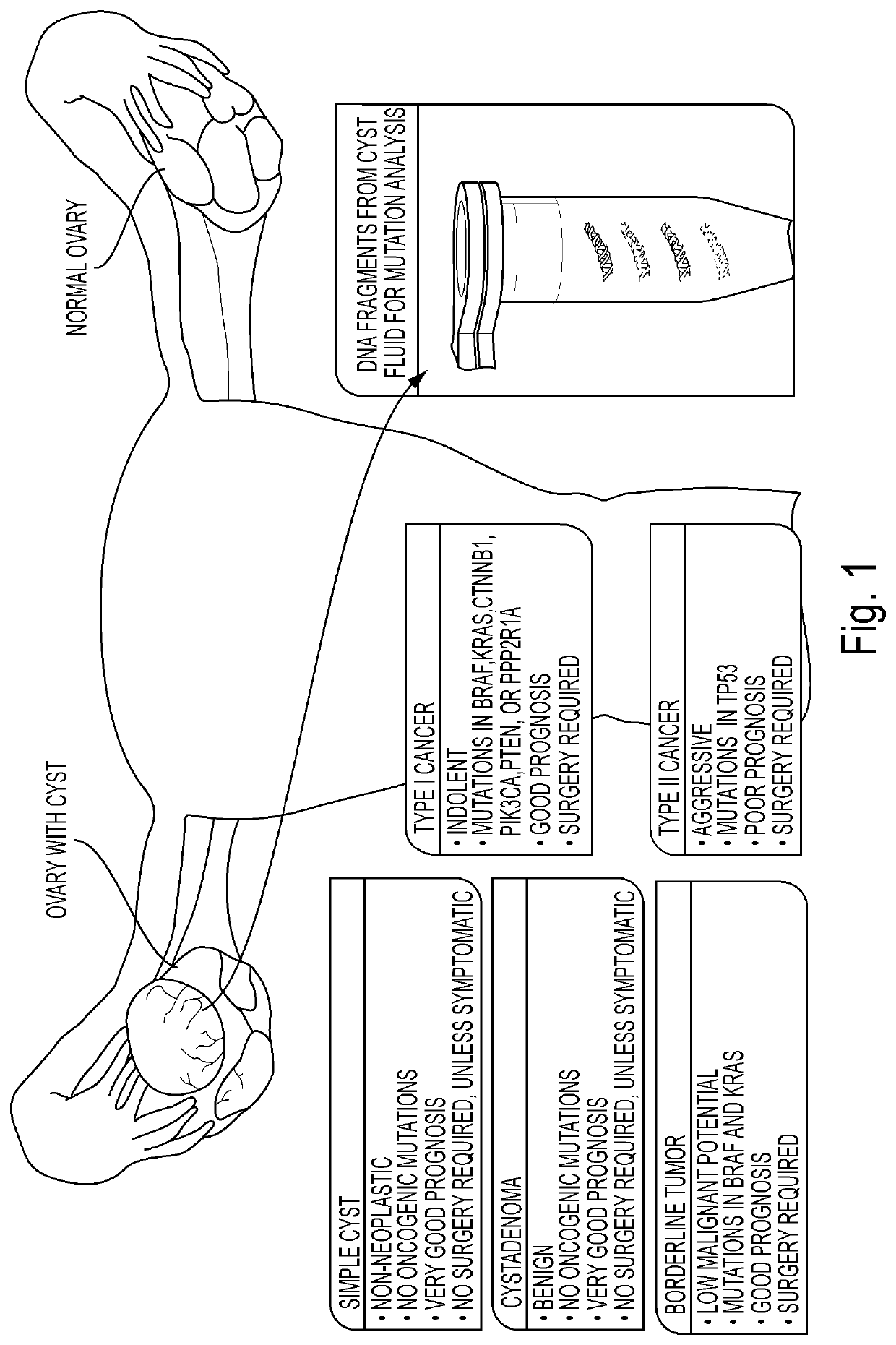
Assaying ovarian cyst fluid
a technology of ovarian cysts and fluids, applied in the field of dna analysis, can solve the problems of serious complications in 15% of women, potentially lethal, and the inability to aspirate ovarian cyst fluid for cytology, and achieve the effect of not being a standard-of-care aspiration method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Patient Samples
[0048]Cyst fluids were collected prospectively from 77 women presenting with a suspected ovarian tumor. Patients were diagnosed by transvaginal sonography or computed tomography and admitted for surgical removal of the cyst by gynecologic oncology surgeons at Sahlgrenska University Hospital, Gothenburg, Sweden. The study was approved by the ethical board of Gothenburg University and patients provided written consent. According to the approved protocol, ovarian cyst fluids were collected after removal of the cyst from the abdomen. All samples were immediately put in 4° C. for 15-30 minutes, centrifuged for 10 minutes at 500 g, and aliquoted into Eppendorf tubes. The fluids were transferred to −80° C., within 30-60 minutes after collection. All histology was reviewed by board-certified pathologists (Table 1).
[0049]Plasma HE4 concentrations were determined using a commercial HE4 EIA assay (Fujirebio Diagnostics) and plasma CA-125 levels were measured...
example 2
Characteristics of the Tumors and Cyst Fluid Samples
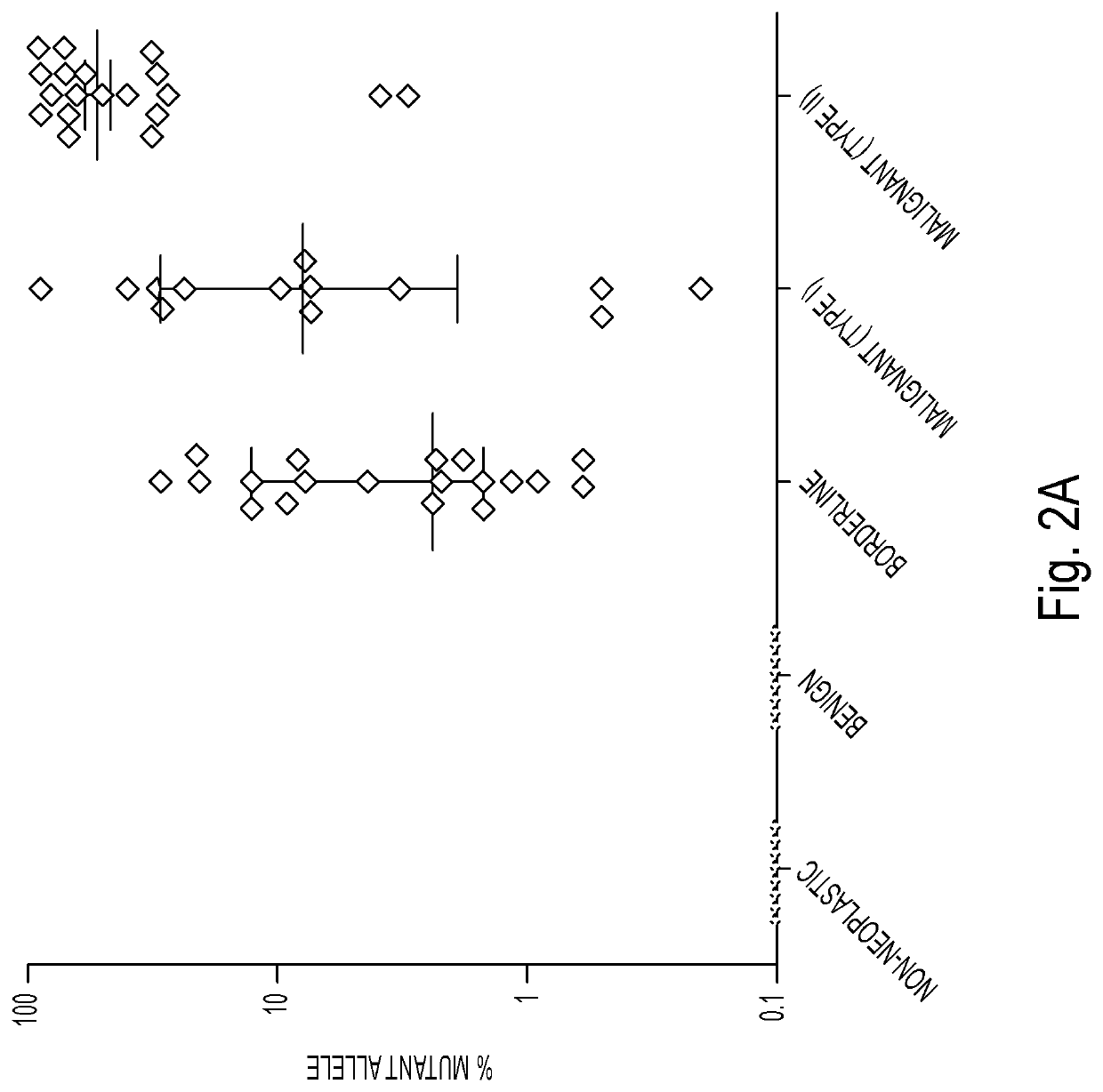
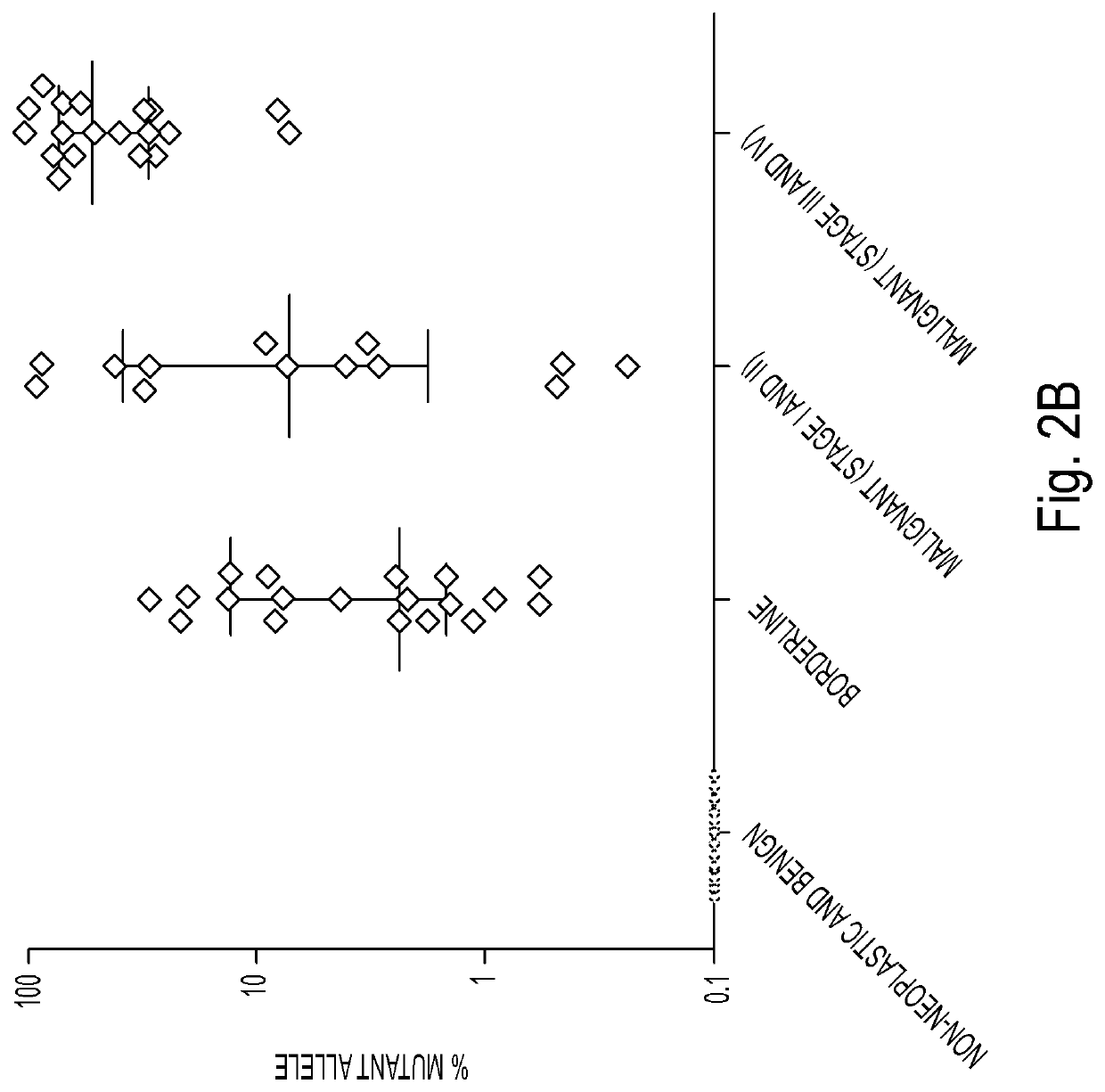
[0053]DNA was isolated from surgically excised ovarian cysts of 77 women. Ten of them had non-neoplastic cysts, 12 had benign tumors, 24 had borderline tumors, and 31 had cancers (13 Type I and 18 Type II). Age, histopathologic diagnosis, stage, and other clinical information are provided in Table 1. The median amount of DNA recovered from the cysts was 222 ng (interquartile range (IQR) of 53 to 3120 ng) (Table 2). There was no significant difference in the amounts of DNA between borderline tumors and type I or type II cancers (Table 2). However, the borderline tumors and cancers contained significantly more DNA than the non-neoplastic cysts or benign tumors (4453±6428 ng vs. 62±64 ng; p<0.001, Wilcoxon rank-sum test).
example 3
A Multiplex PCR-Based Test to Identify Tumor-Specific Mutations in Cyst Fluid Samples
[0054]We designed a multiplex PCR-based test that could simultaneously assess the regions of 17 genes frequently mutated in ovarian tumors. The amount of DNA shed from neoplastic cells was expected to be a minor fraction of the total DNA in the cyst fluid, with most DNA emanating from normal cells. We therefore used a sensitive detection method, called Safe-SeqS (Safe-Sequencing System), to identify mutations in cyst fluid samples (34). In brief, primers were designed to amplify 133 regions, covering 9054 distinct nucleotide positions within the 17 genes of interest (Table S1). Three multiplex PCR reactions, each containing non-overlapping amplicons, were then performed on each sample. One primer in each pair included a unique identifier (UID) for each template molecule, thereby drastically minimizing the error rates associated with PCR and sequencing, as described previously (34) (Table S1). Under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com