Broad specificity affinity arrays: a qualitative approach to complex sample discrimination
a broad specificity and array technology, applied in the field of broad specificity affinity arrays, can solve the problems of series of complex global modifications in the composition and thereby the structure of carbohydrates, which are currently not possible, and achieve the effect of increasing the informational content and achieving the signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
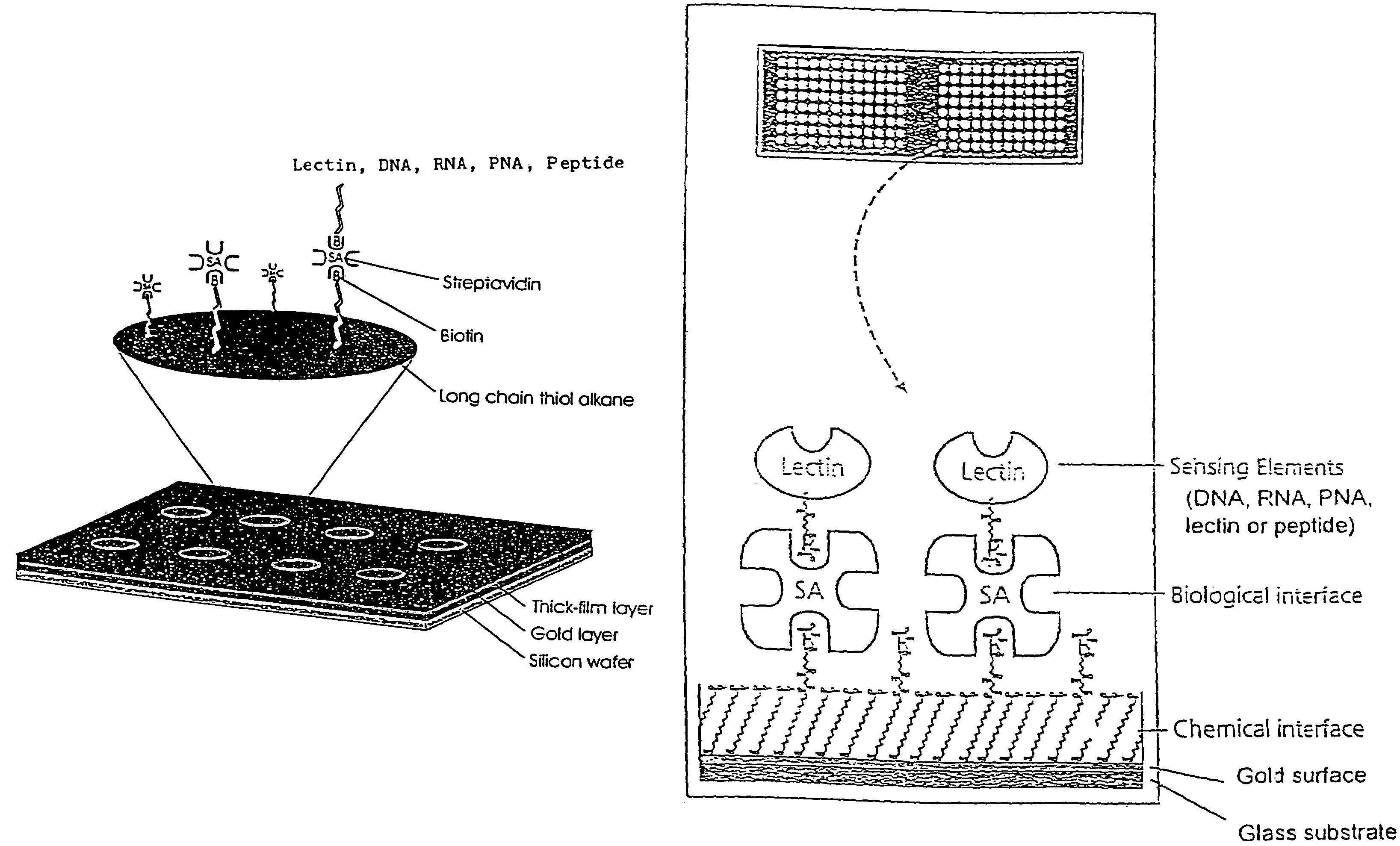
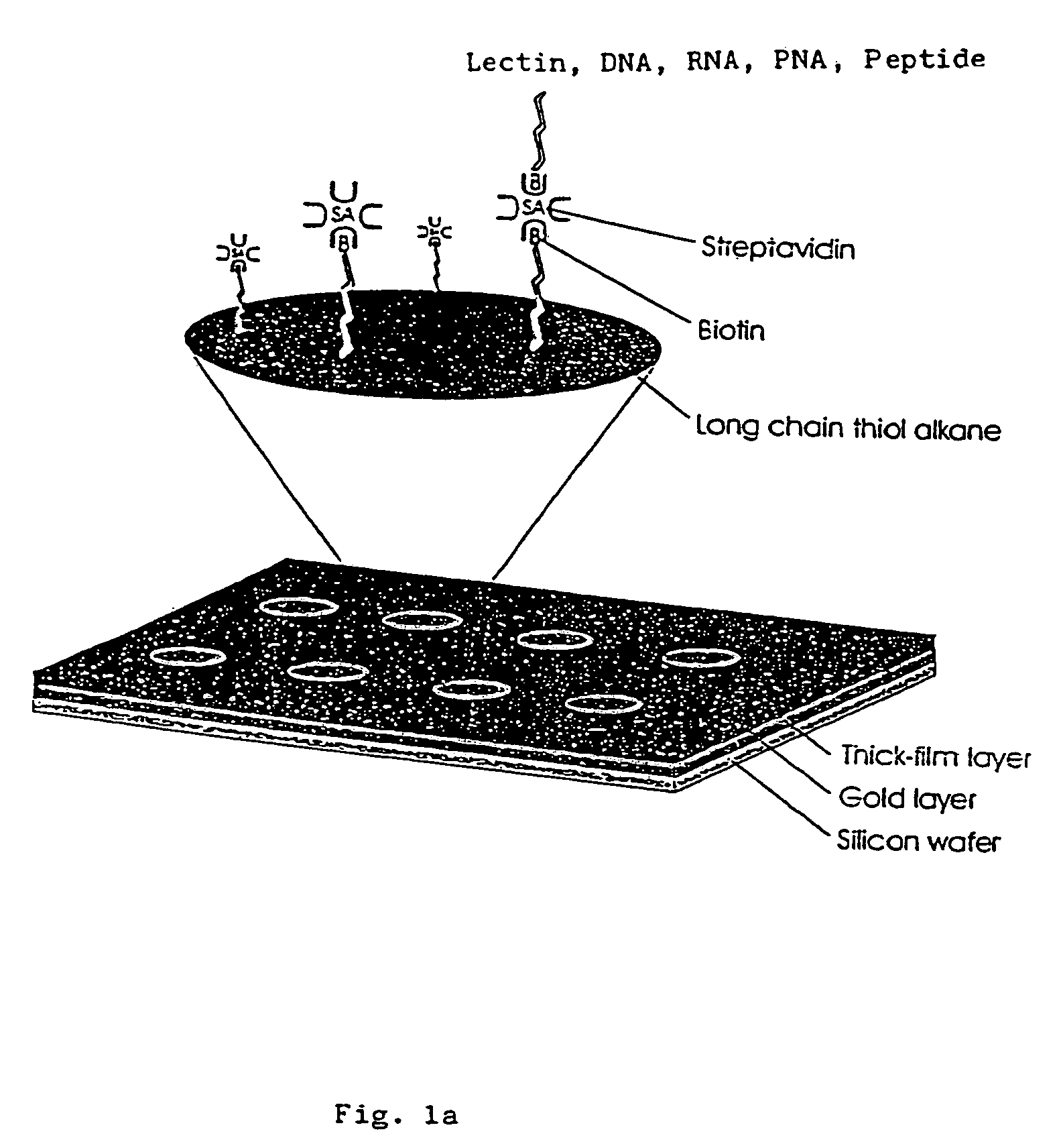
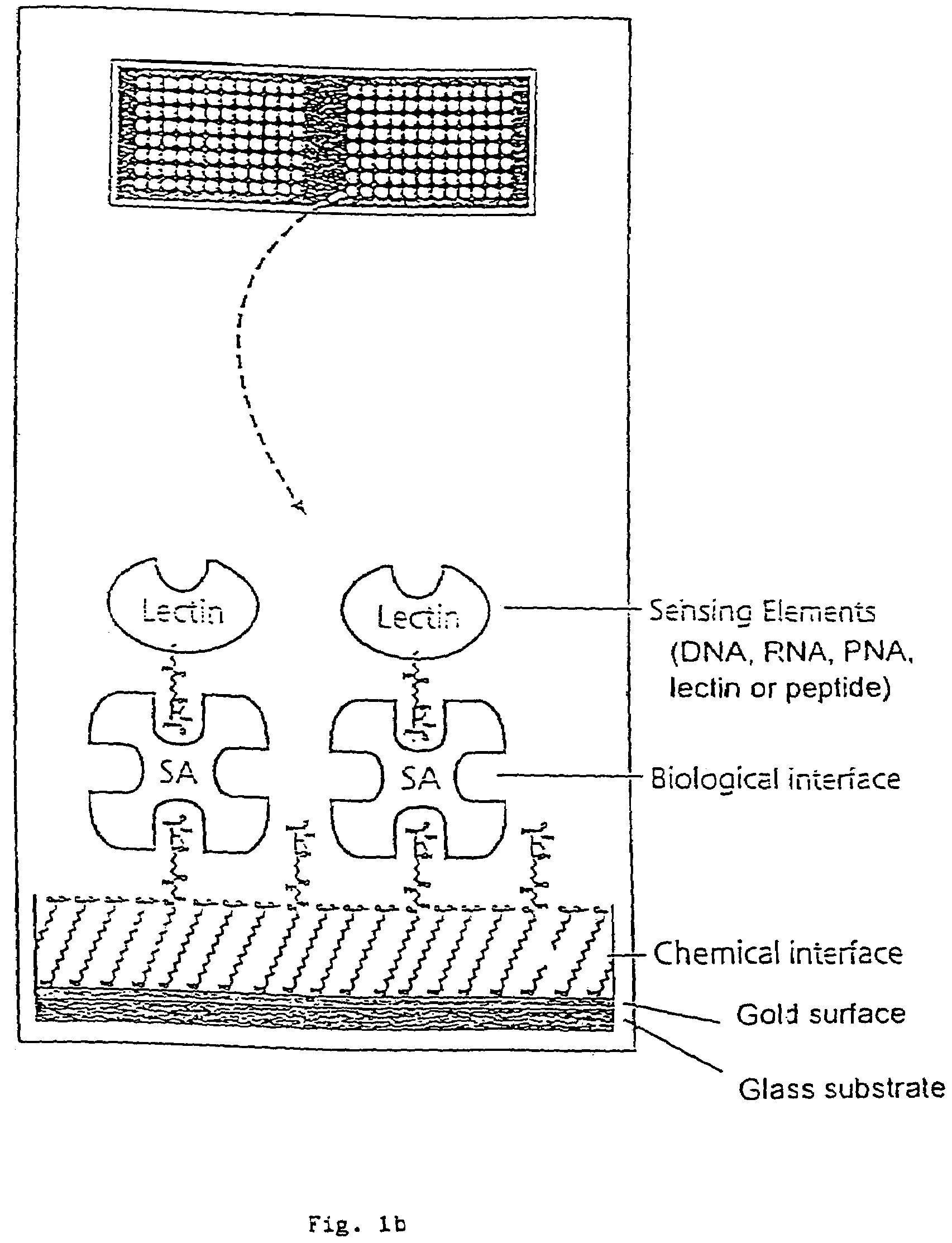
[0048]Interfacing these biological sensing elements with the surface mass based optical imaging technology was very difficult. Standard immobilization protocols resulted in poor overall reproducibility and lead us to develop a highly specialized protocol which combines surface patterning and immobilization technologies (FIG. 1). The integrated assay format which combines thick film surface patterning, self-assembling monolayers, efficient coupling chemistries and the biotin-streptavidin. The procedure employs a proprietary teflon based thick-film printing ink (Cell-line, USA) to pattern gold coated silicon wafers or glass combined with self-assembling carboxyl-terminated long chain thiol alkanes onto the exposed gold surfaces. Polished silicon wafers (Wacker Chemie, Germany) or glass were coated with gold by evaporation as described (Mårtensson, J., Arwin, H. Intepretation of spectroscopic ellipsometric data on protein layers on gold including substrate-layer interactions. (1995) La...
example 2
[0052]In these studies, sick vs healthy human serum samples were analysed using the same array of eight biotinylated lectins: canavalia ensiformis, bandeiraea simplicifolia BS-I, arachis hypogaea, phytolacca americana, phaseolus vulgaris pha-e, artocarpus integrifolia, triticum vulgaris, pisum sativum. In this case, unpatterned gold (50 nm thick gold evaporated by sputtering) coated glass (0.3 mm thick glass) surfaces were prepared essentially as described above up to and including the coupling of amino-biotin. The surfaces were then inserted into the BIAcore from Pharmacia Biosensor. The running conditions were 2 μl / min, at 25° C. and the running buffer was HBST. The binding of the SA and biotinylated lectins was performed by sequentially injecting 4 μl of a 50 μg / ml solution of each.
[0053]The human sera were obtained from the Infectious Diseases Department at Lunds University Hospital. The reference sera were taken from healthy volunteers (20 individuals). The sick sera samples (8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com