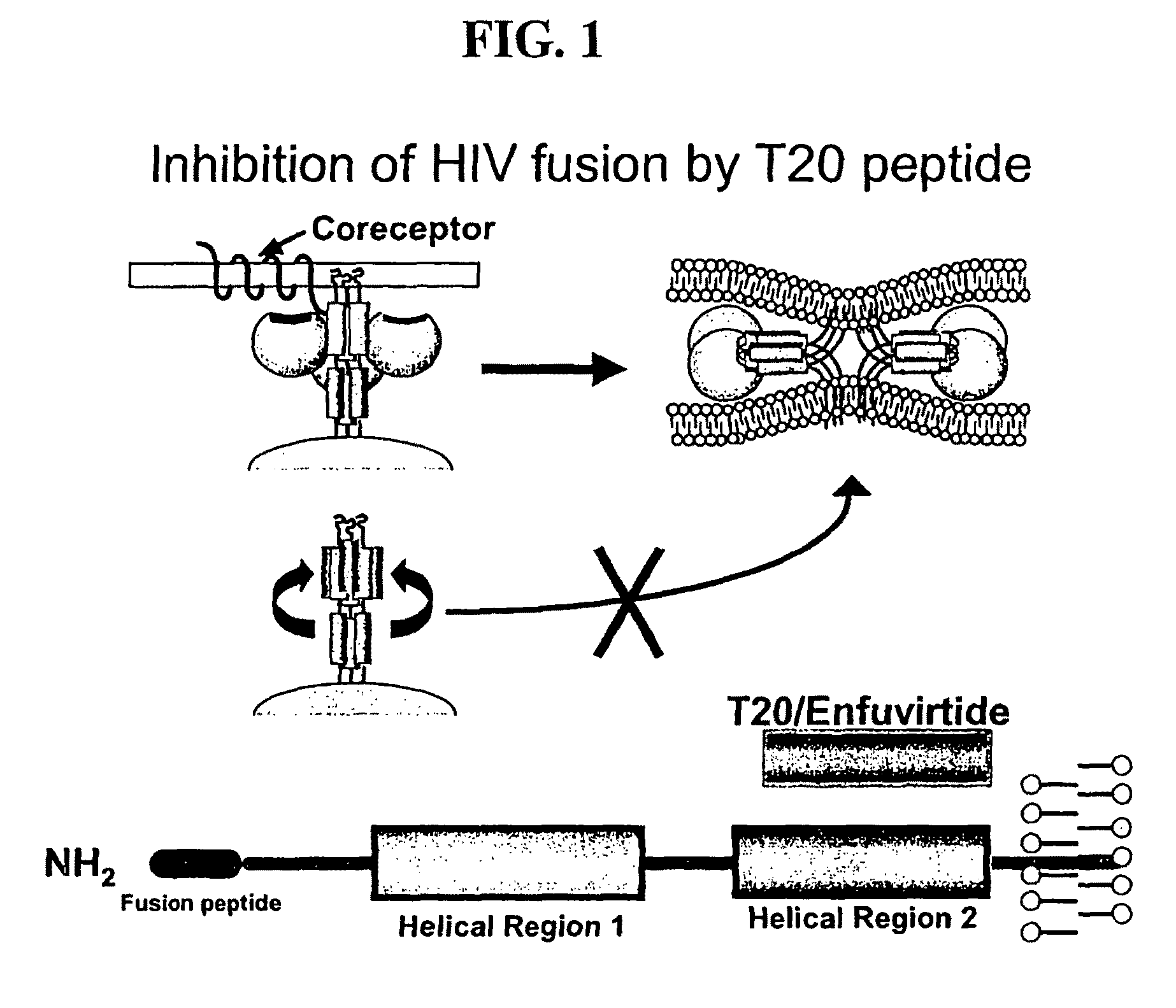
Inhibition of membrane fusion proteins
a technology of membrane fusion and protein, which is applied in the field of viral infection control, can solve the problems of insufficient antiviral strategies and sorely needed alternative strategies, and achieve the effects of inhibiting infection, and reducing quenching of fluorescent molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Domain III from Class III Fusion Proteins Functions as a Dominant-Negative Inhibitor of Virus Membrane Fusion
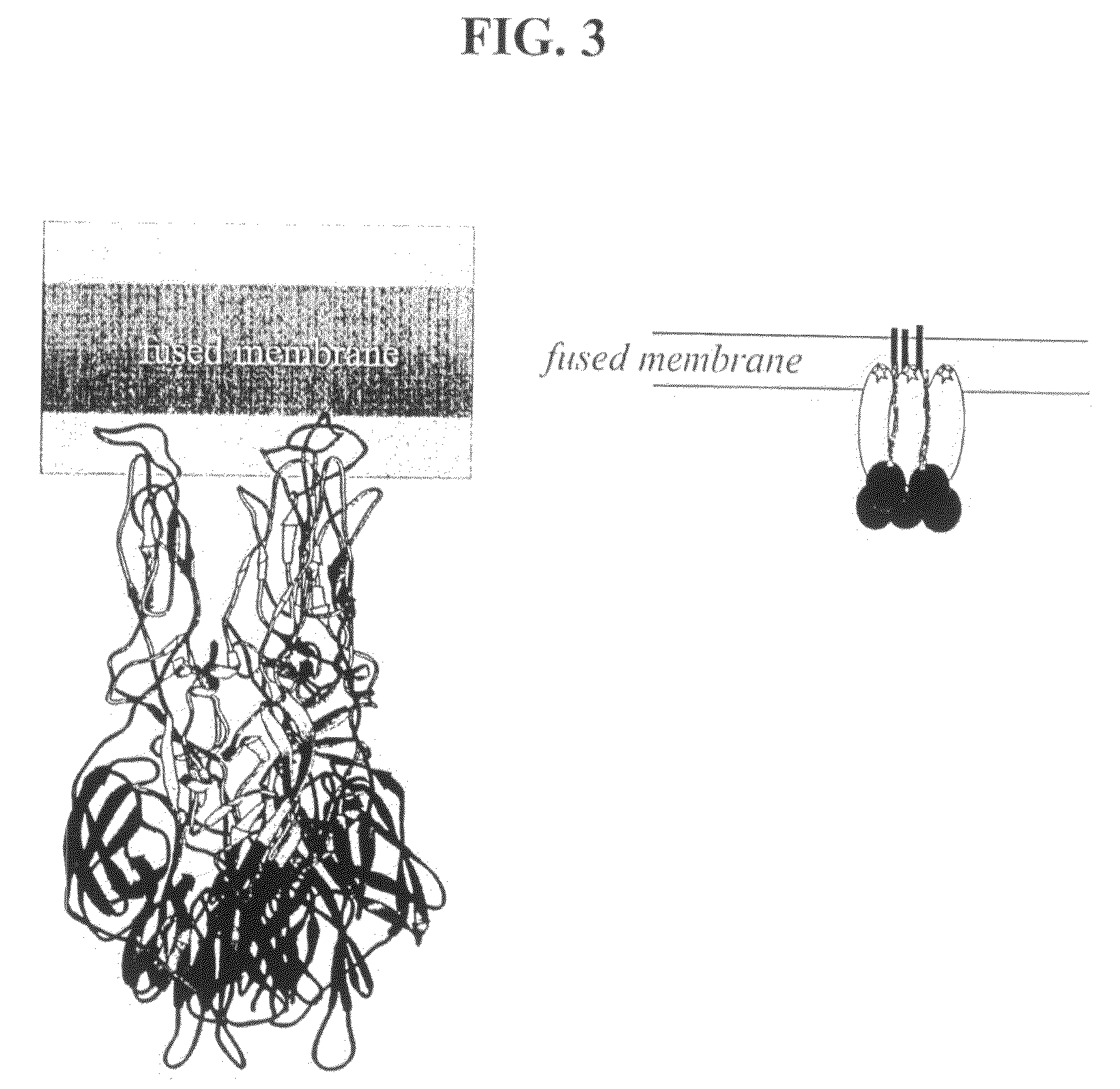
[0186]Rationale and domain III expression. The structure of the class II homotrimer suggests several features that might be targets for inhibition of the fusion reaction. The pH 7 form of the dengue virus E protein crystallized with a molecule of detergent bound in a hydrophobic pocket near the “hinge” region between domains 1 and II (Modis et al., 2003). As the hinge changes its angle during the transition to the trimer form, inhibition of this flexibility may block fusion and infection. Thus, molecules that dock into the hydrophobic pocket are potential antivirals (Modis et al., 2003). The structure of the fusion protein homotrimer reveals that the “stem” region of the protein interacts (or would be predicted to interact in the case of the dengue HT and TBE HT, which do not contain the stem) with an HT core region composed of domains I and II (see stem in FIG. 3 schematic)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com