Traditional Chinese medicine active part compound preparation for curing cardiac and cerebral vascular diseases and its preparing method
A technology for cardiovascular and cerebrovascular diseases and effective parts, applied in the field of medicine, can solve problems such as unclear ingredients, unstable curative effect, and unknown mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
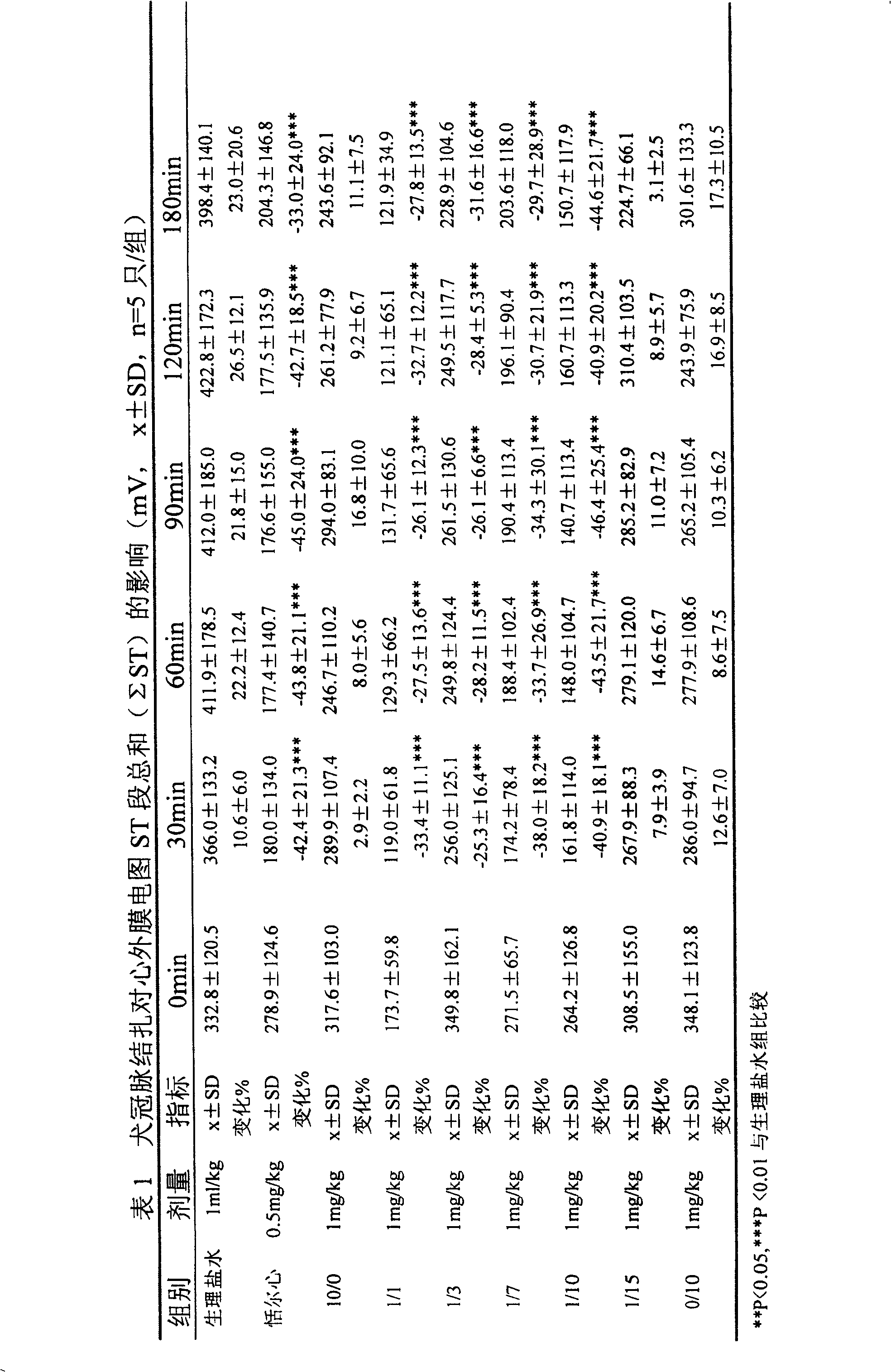
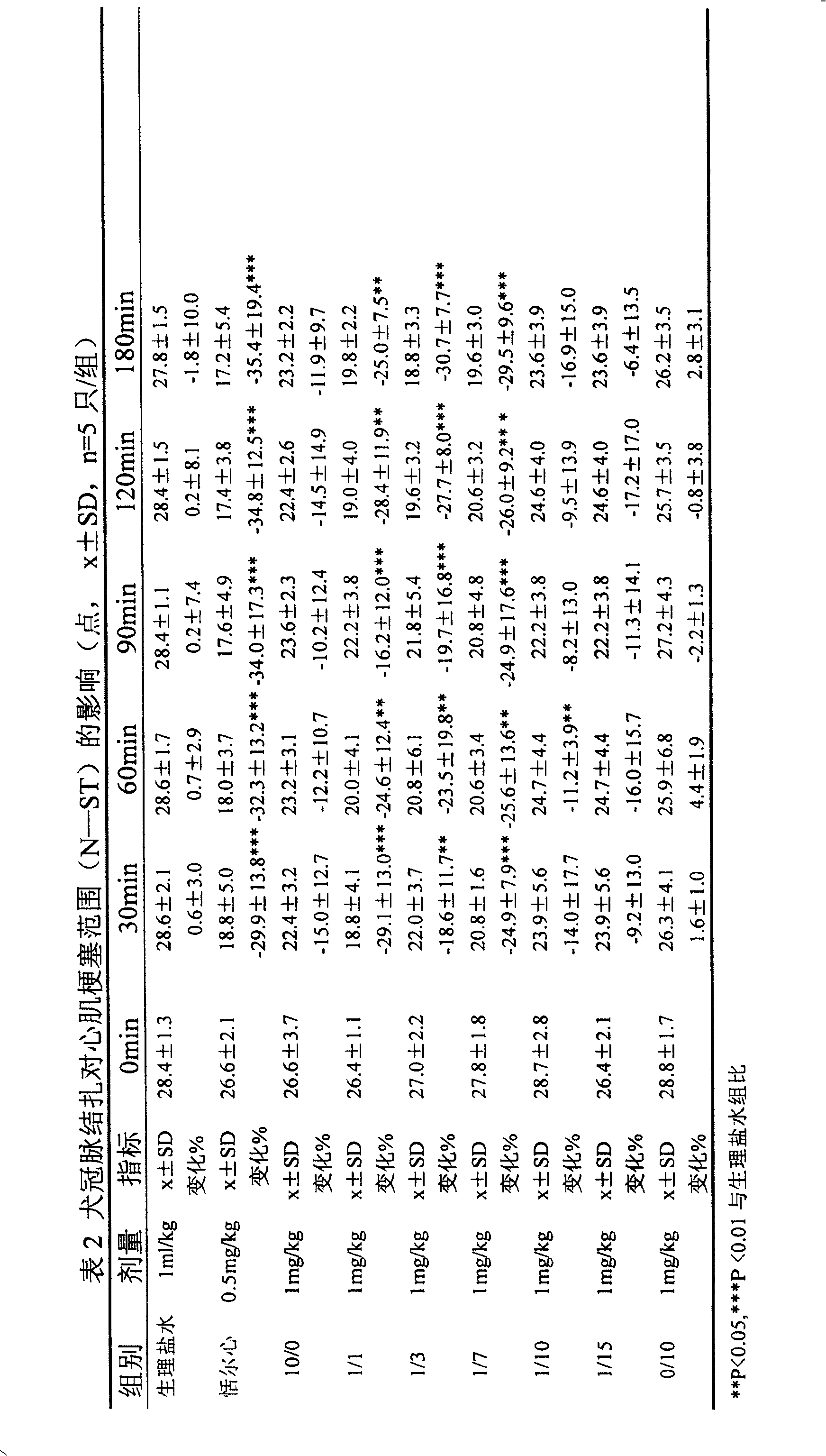
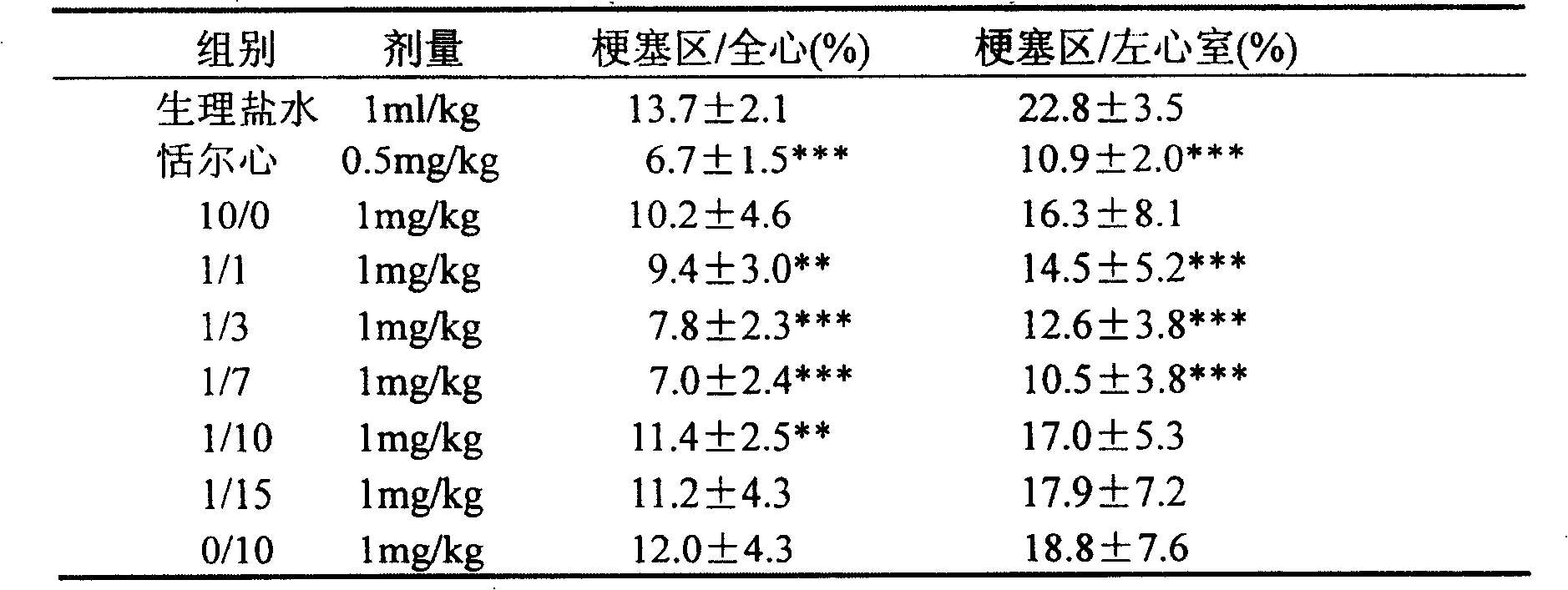
[0039]Get 10kg of Salvia miltiorrhiza, soak 30min with 70% acetone of 10 times the amount and start percolation, the percolation solution is concentrated under reduced pressure to no acetone smell, dilute the concentrated solution with an appropriate volume of water, according to the ratio of adsorption capacity 1: 1 (resin content and the amount of medicinal material) through the AB-8 macroporous resin column, that is, the column is filled with 10kg macroporous resin. First eluted with 4 times the volume of deionized distilled water, then eluted with 10 times the column volume of 20% ethanol solution, and collected the eluate to obtain the salvianolic acid component. Concentrate under reduced pressure at 40°C to remove ethanol, continue to concentrate to 1 / 10 of the original volume, filter through a 0.45 microporous membrane, and freeze-dry the concentrated solution according to the following process: -50°C for 4 hours, -10°C for lyophilization, and warm to 20 ° C for 3 hours...
Embodiment 2
[0043] Get 10kg of Salvia miltiorrhiza, soak with 10 times the amount of 50% acetone for 30min and then start percolating. The percolating liquid is concentrated under reduced pressure until there is no acetone smell, dilute the concentrated solution with an appropriate volume of water, and use a ratio of 1:1 (resin content) and the amount of medicinal material) through the AB-8 macroporous resin column, that is, the column is filled with 10kg macroporous resin. First eluted with 4 times the volume of deionized distilled water, then eluted with 12 times the column volume of 30% ethanol solution, and collected the eluate to obtain the salvianolic acid component. Concentrate under reduced pressure at 45°C to remove ethanol, continue to concentrate to 1 / 10 of the original volume, filter through a 0.45 microporous membrane, and freeze-dry the concentrate according to the following process: -50°C for 3 hours, -10°C for 3 hours, and heat up to Keep warm at 20°C for 2 hours, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com