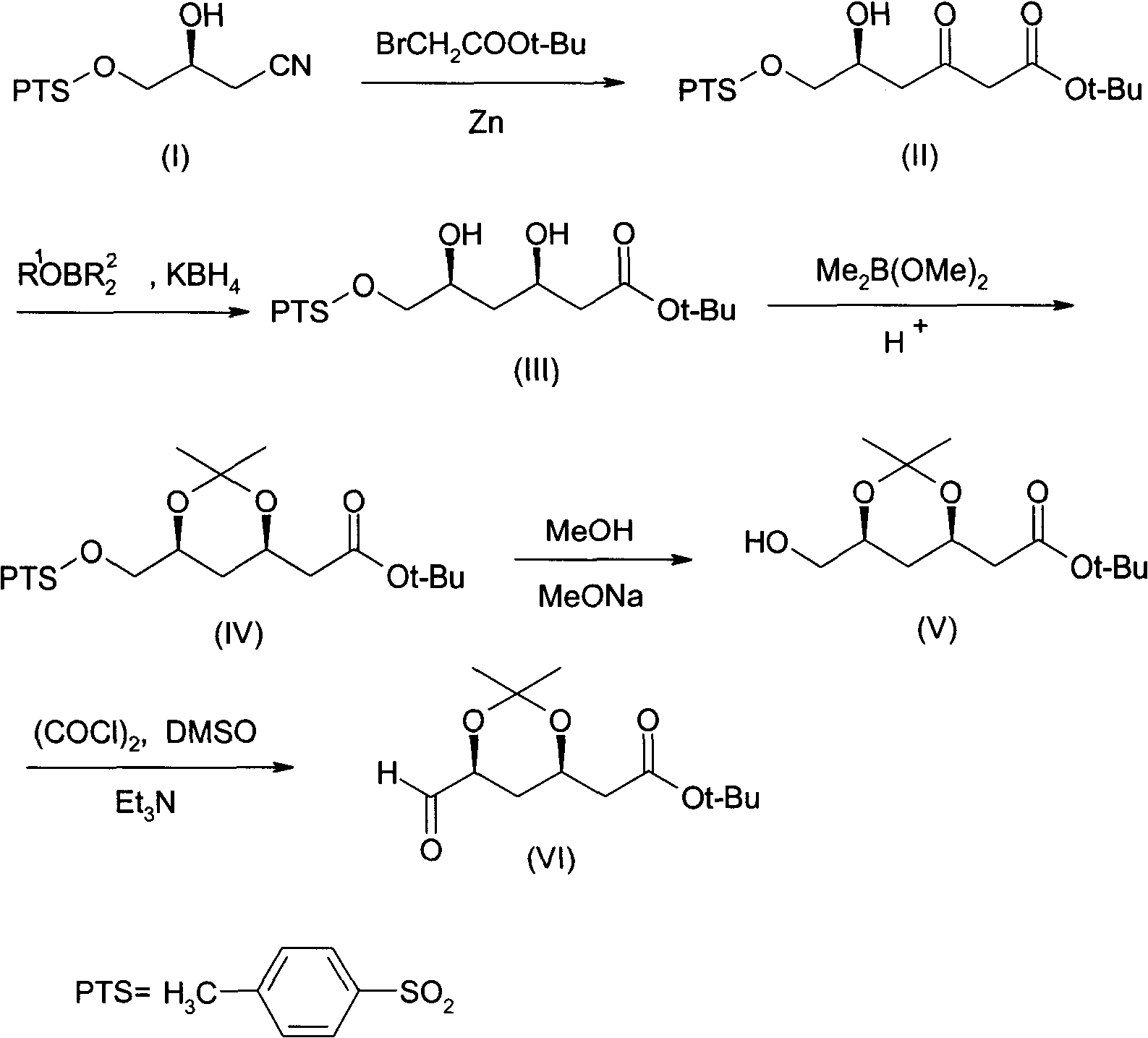
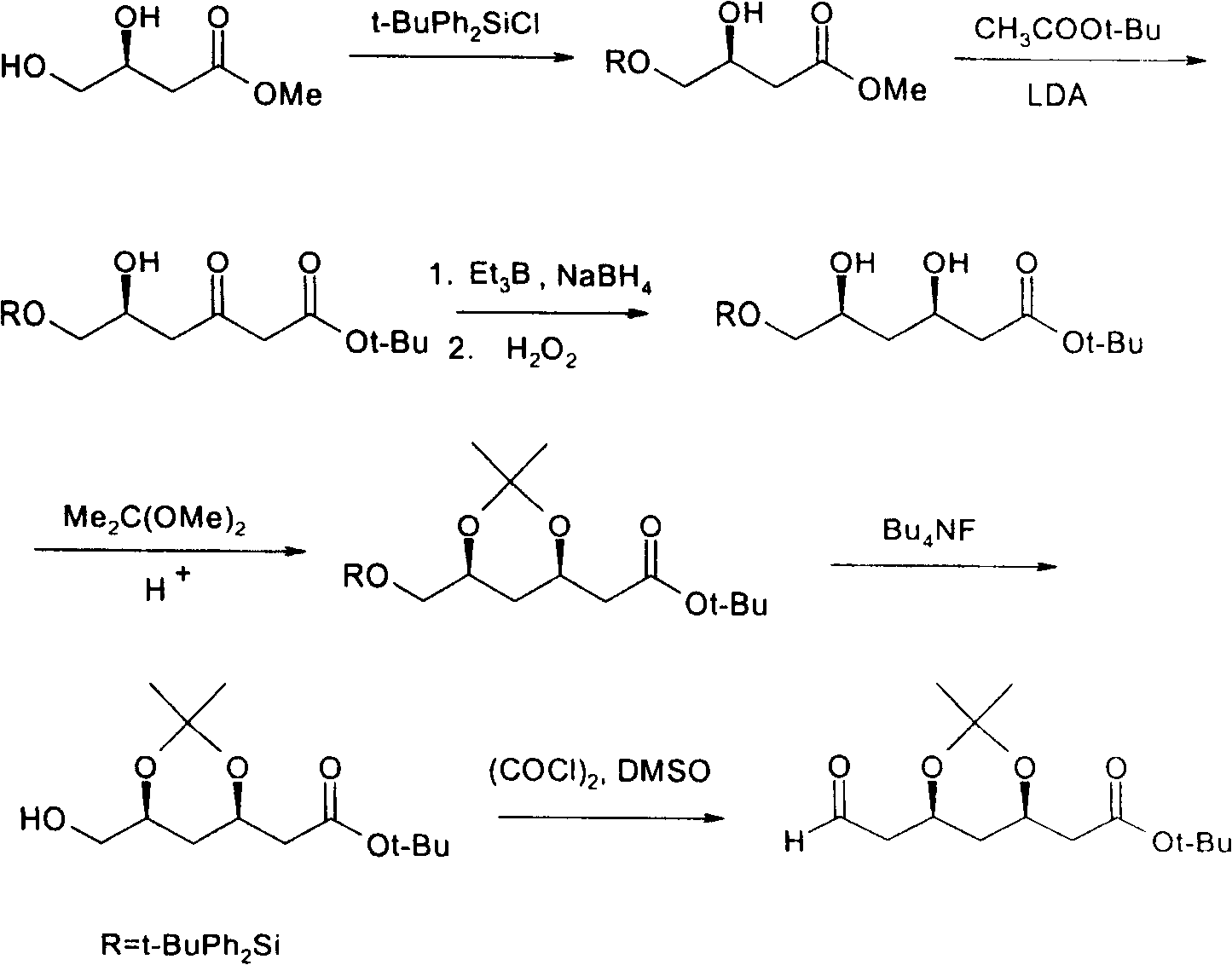
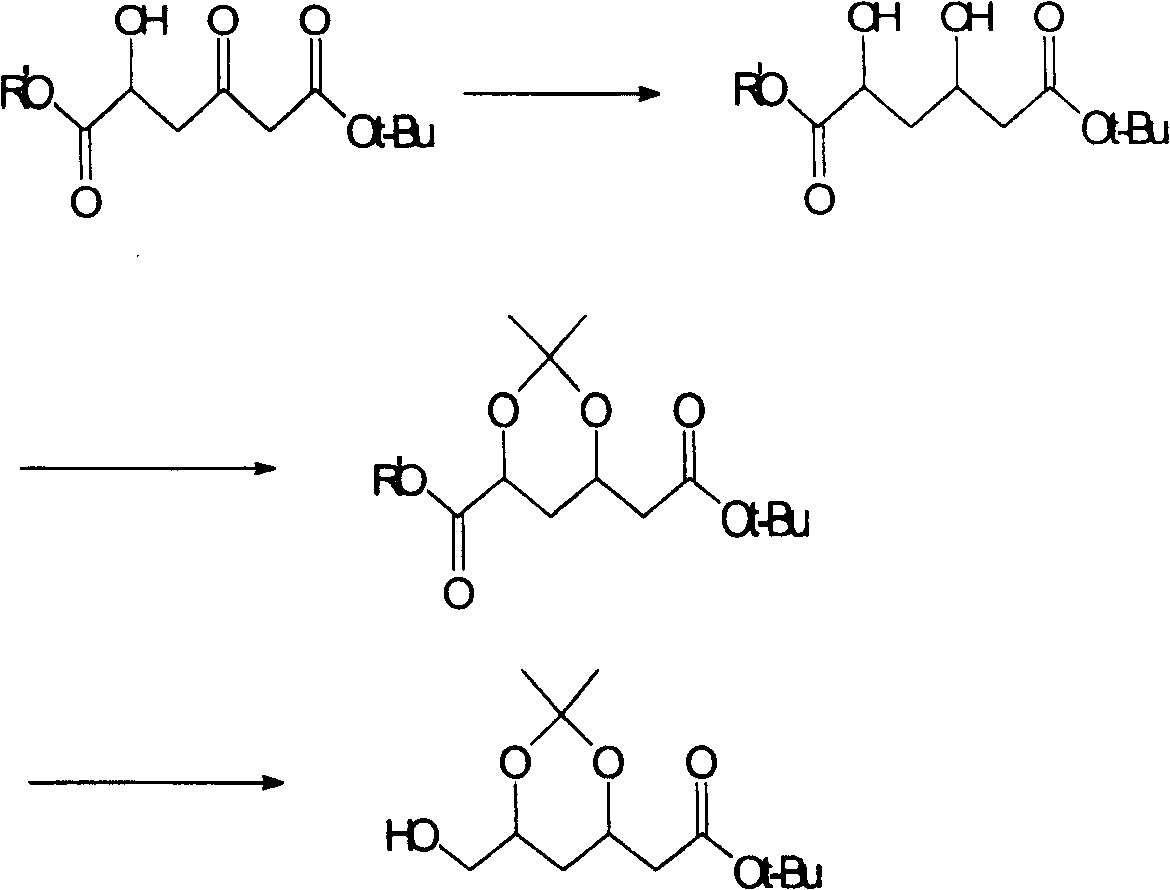
(4R-cis)-6-formyl-2,2-dimethyl-1,3- dioxane -4- tertiary butyl acetate synthesis method
A technology of tert-butyl acetate and dioxane, applied in directions such as organic chemistry, can solve problems such as quantitative analysis of intermediates and difficulty in purification, and achieve the effects of improving purity, short process and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Synthesis of (5S)-5-hydroxy-6-p-toluenesulfonyloxy-3-oxohexanoic acid tert-butyl ester (II)
[0051]Add 65g of zinc foil, 400mL of tetrahydrofuran, and 0.7g of CuBr catalyst into a three-neck flask, stir and raise the temperature to 35°C, add 195g of tert-butyl bromoacetate dropwise, and keep the reaction for 1 hour after dropping. After the incubation, add dropwise a solution composed of 255g (3R)-3-hydroxyl-4-p-toluenesulfonyloxybutyronitrile (I) and 400mL tetrahydrofuran (THF). ). Cool to -5°C, add dropwise 10% sulfuric acid solution to adjust pH=3-4, keep stirring for 3 hours. The reaction solution was extracted with ethyl acetate, anhydrous MgSO 4 Dry, concentrate in vacuo to about 400mL, cool to -15°C, insulate and crystallize for 12 hours, filter, and dry in vacuo to obtain (5S)-5-hydroxy-6-p-toluenesulfonyloxy-3-oxohexanoic acid tert-butyl The ester (II) was 228g, and the content measured by HPLC was 99.0%.
[0052] (2) Synthesis of (3R, 5S)-3,5-dihydrox...
Embodiment 2
[0061] (1) Synthesis of (5S)-5-hydroxy-6-p-toluenesulfonyloxy-3-oxohexanoic acid tert-butyl ester (II)
[0062] Add 65g of zinc foil, 400mL of tetrahydrofuran, and 0.7g of CuBr catalyst into a three-neck flask, stir and raise the temperature to 40°C, add 195g of tert-butyl bromoacetate dropwise, and keep the reaction for 1 hour after dropping. After the incubation, add dropwise a solution composed of 255g (3R)-3-hydroxyl-4-p-toluenesulfonyloxybutyronitrile (I) and 400mL tetrahydrofuran (THF). ). Cool to 0°C, add dropwise 10% sulfuric acid solution to adjust pH=3-4, keep stirring for 3 hours. The reaction solution was extracted with ethyl acetate, anhydrous MgSO 4 Dry, concentrate in vacuo to about 400mL, cool to -10°C, insulate and crystallize for 12 hours, filter, and dry in vacuo to obtain (5S)-5-hydroxy-6-p-toluenesulfonyloxy-3-oxohexanoic acid tert-butyl The ester (II) was 228g, and the content measured by HPLC was 99.2%.
[0063] (2) Synthesis of (3R, 5S)-3,5-dihydrox...
Embodiment 3
[0072] This example is the synthesis of (3R,5S)-3,5-dihydroxy-6-p-toluenesulfonyloxyhexanoic acid tert-butyl ester (III).
[0073] Add 186g (5S)-5-hydroxy-6-p-toluenesulfonyloxy-3-oxohexanoic acid tert-butyl ester (II), 500mL tetrahydrofuran and methanol mixed solvent into the three-necked flask, cool to -35°C, Add 300 mL of 2 mol / L ethoxydiethylborane, and keep the reaction for 2.5 hours. Cool down to -75°C, add KBH 4 30g, stir the reaction until the reaction of (II) is complete (HPLC tracking), raise the temperature to 0-10°C, add glacial acetic acid to neutralize to pH4 After drying, ethyl acetate was removed by distillation under reduced pressure to obtain 183 g of (3R,5S)-3,5-dihydroxy-6-p-toluenesulfonyloxyhexanoic acid tert-butyl ester (III), which was measured by HPLC chiral column ( 3R, 5S) isomer (III) content was 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com