Freeze dried powder injection of tramadol hydrochloride and its preparation process
A technology of tramadol hydrochloride and freeze-dried powder injection, which can be used in freeze-dried delivery, powder delivery, pharmaceutical formulation and other directions, can solve the problems of inconvenient transportation and portability, poor stability, etc., and achieves stability, porosity, and formability. Good, easy to transport effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Raw materials and dosage
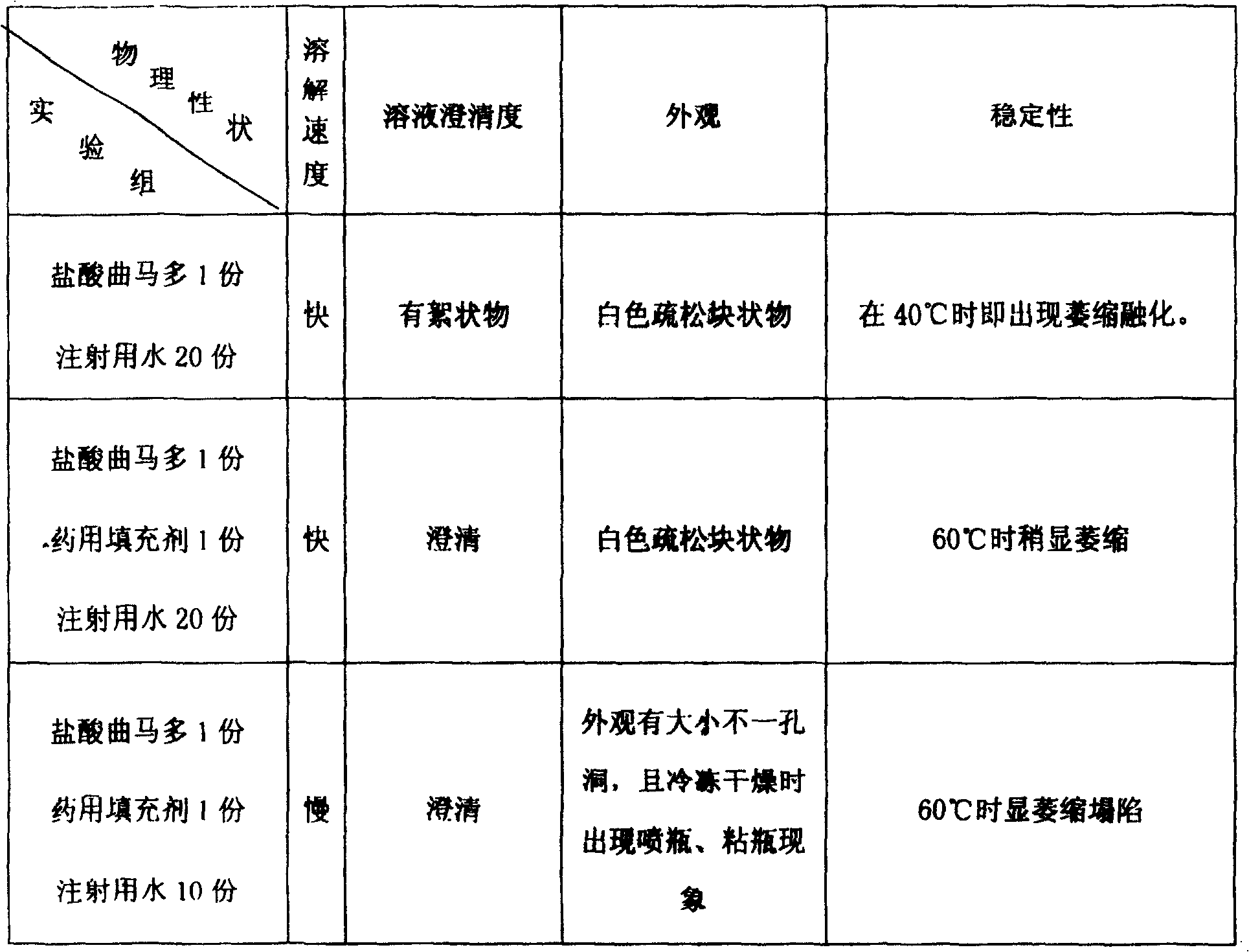
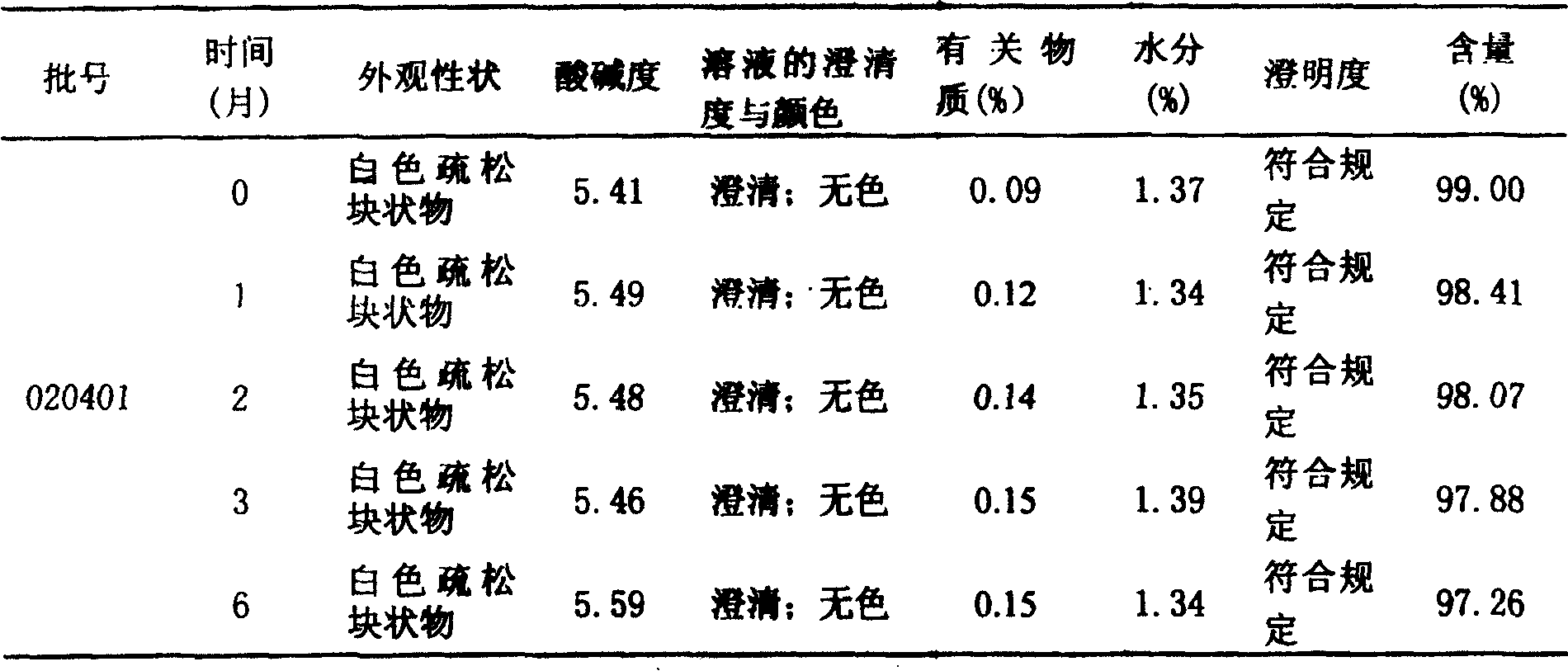
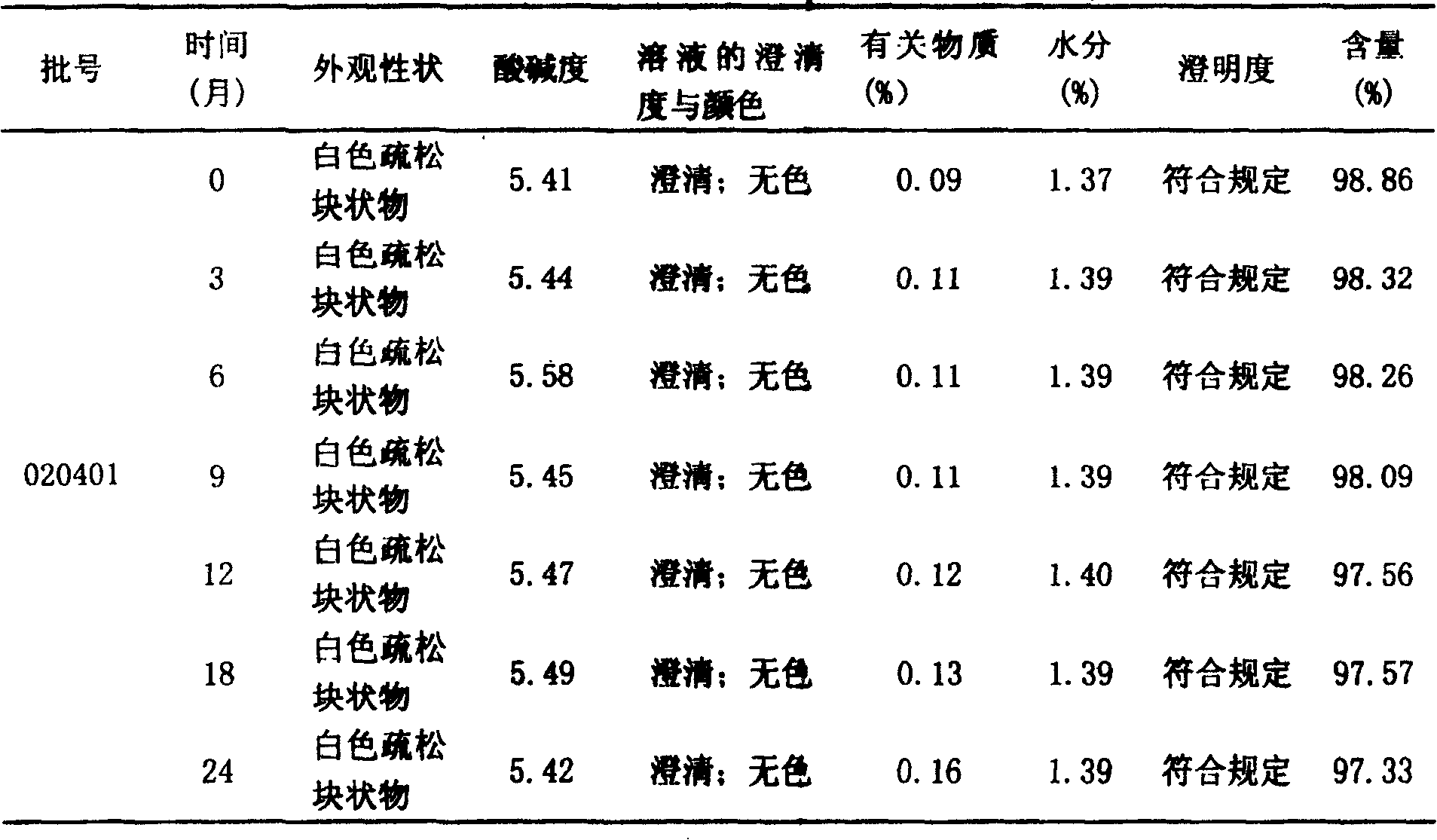
[0051] 1000 grams of tramadol hydrochloride and 1000 grams of mannitol, take the above tramadol hydrochloride and mannitol, add 16000ml of water for injection, stir at high speed until dissolved, adjust the pH value to 6.0, add 1‰ of activated carbon and stir for about 30 minutes, and Filter the solution, remove gac, get an appropriate amount of solution and measure the content according to the method under the content item of tramadol hydrochloride in the Pharmacopoeia in 2000, add water for injection to determine the volume to about 20000ml according to the actual measurement content (contain tramadol hydrochloride 50mg to the volume in 1ml), first use Filter twice with 0.45 μm cellulose membrane, and then filter twice with 0.22 μm cellulose membrane, then prepare for canning;
[0052] Debug the canning machine, and fill it according to the theoretical content of 100% (1ml).
[0053] Put the canned samples into the freeze dryer, first pre-f...
Embodiment 2
[0056] Raw materials and dosage
[0057] 1000 grams of tramadol hydrochloride and 1500 grams of glucose, take the above tramadol hydrochloride and glucose, add 16000ml of water for injection, stir at high speed until dissolved, adjust the pH value to 6.0, add 1‰ of activated carbon and stir for about 30 minutes, dissolve the solution Filter, remove gac, get appropriate amount of solution and measure content by the method under the content item of tramadol hydrochloride in Pharmacopoeia 2000, add water for injection to determine the volume about 25000ml according to the actual determination content (according to 1ml containing tramadol hydrochloride 50mg volume), first use 0.45 After filtering twice with a μm cellulose membrane, and then twice with a 0.22 μm cellulose membrane, prepare for canning;
[0058] Debug the canning machine, and fill it according to the theoretical content of 100% (1ml).
[0059] Put the canned samples into the lyophilizer, first pre-freeze the sample...
Embodiment 3
[0062] Raw materials and dosage
[0063]Tramadol hydrochloride 1000g, sodium lactobionate 500g, take the above tramadol hydrochloride and sodium lactobionate, add 16000ml water for injection, stir at high speed until dissolved, adjust the pH value to 6.0, add 1‰ of activated carbon and stir for about 30 Minutes, filter the solution, remove gac, get an appropriate amount of solution and measure the content according to the method under the content item of tramadol hydrochloride in the Pharmacopoeia in 2000, add water for injection to make up about 20000ml according to the actual measured content (according to 1ml containing tramadol hydrochloride 50mg constant volume) , first filter twice with a 0.45μm cellulose membrane, then filter twice with a 0.22μm cellulose membrane, and prepare for canning;
[0064] Debug the canning machine, and fill it according to the theoretical content of 100% (1ml).
[0065] Put the canned sample into the freeze dryer, first pre-freeze the sample ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com