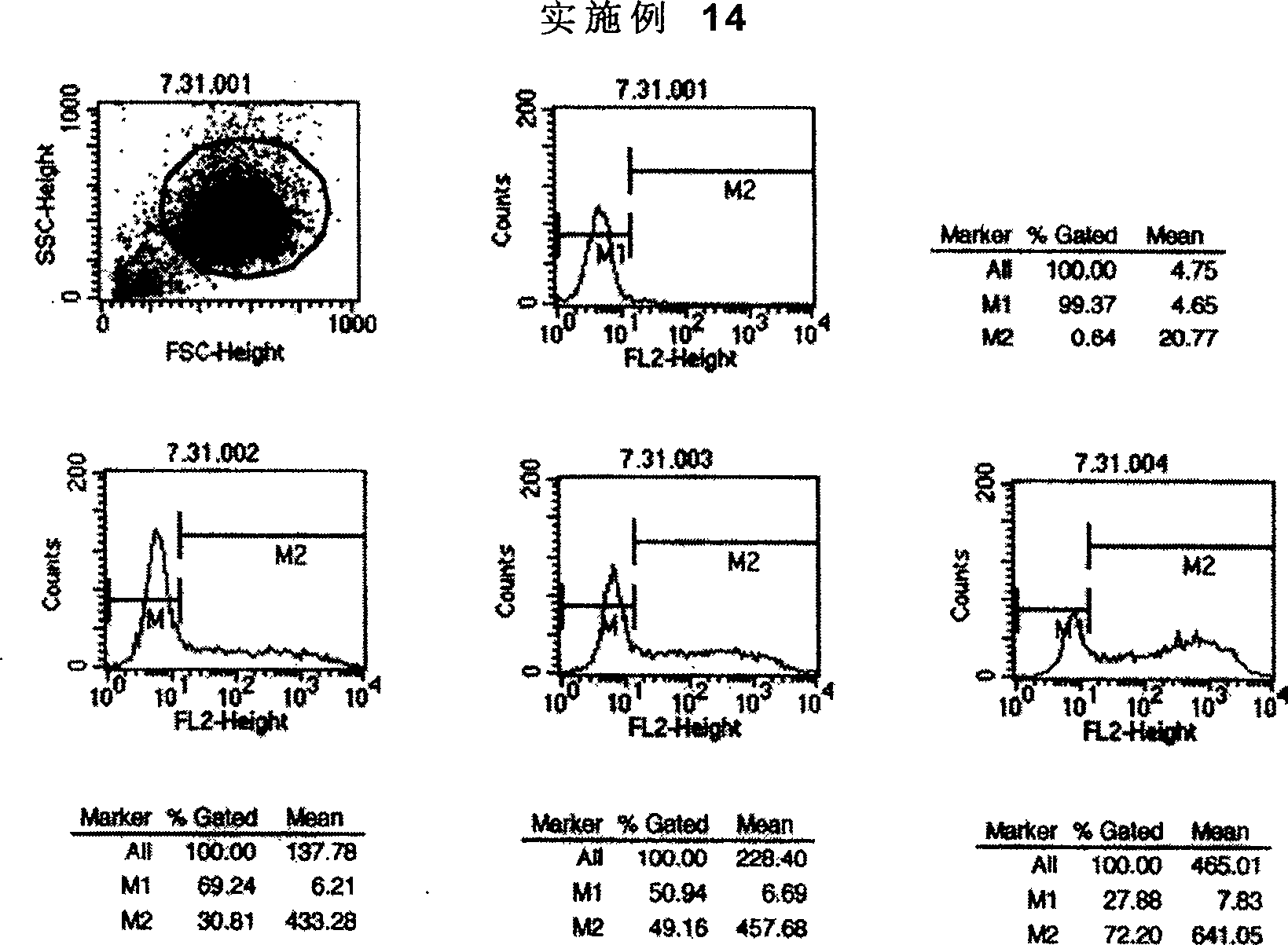
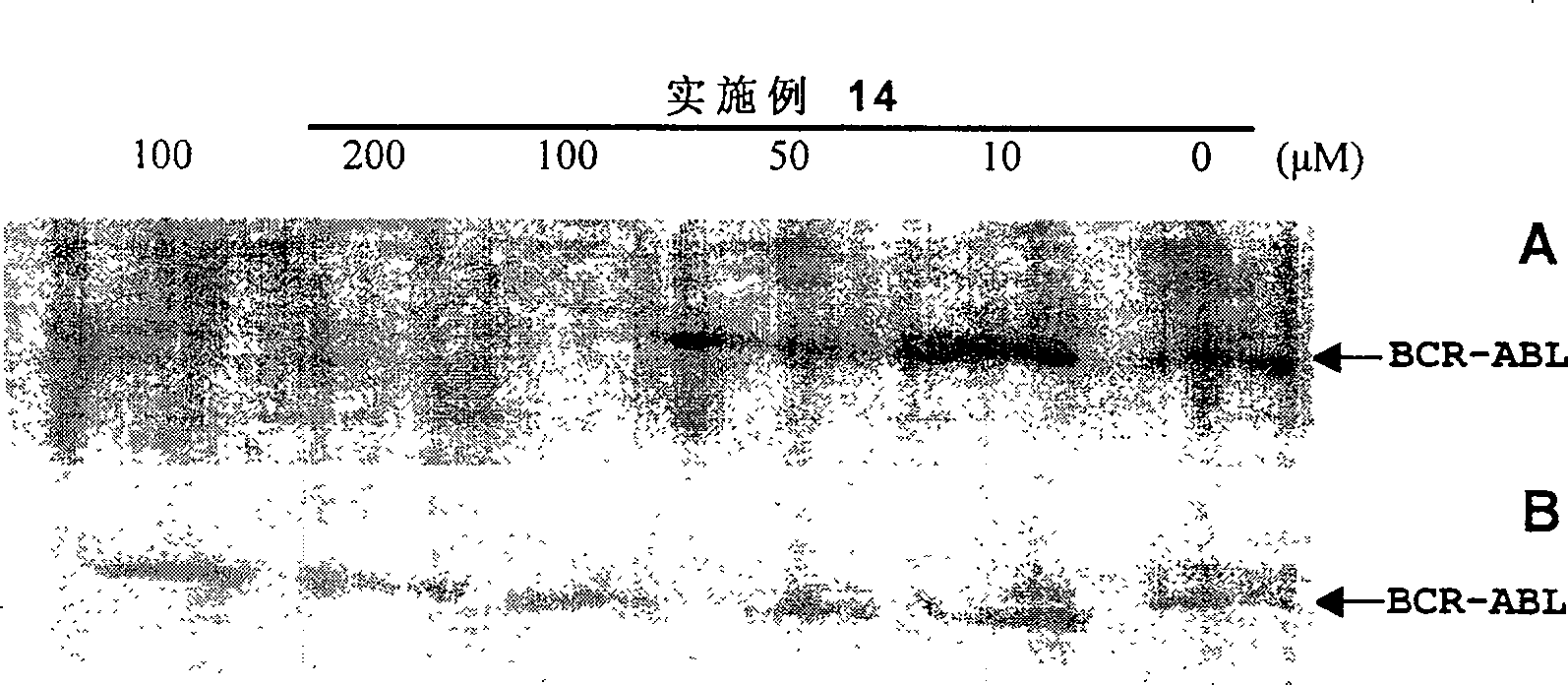
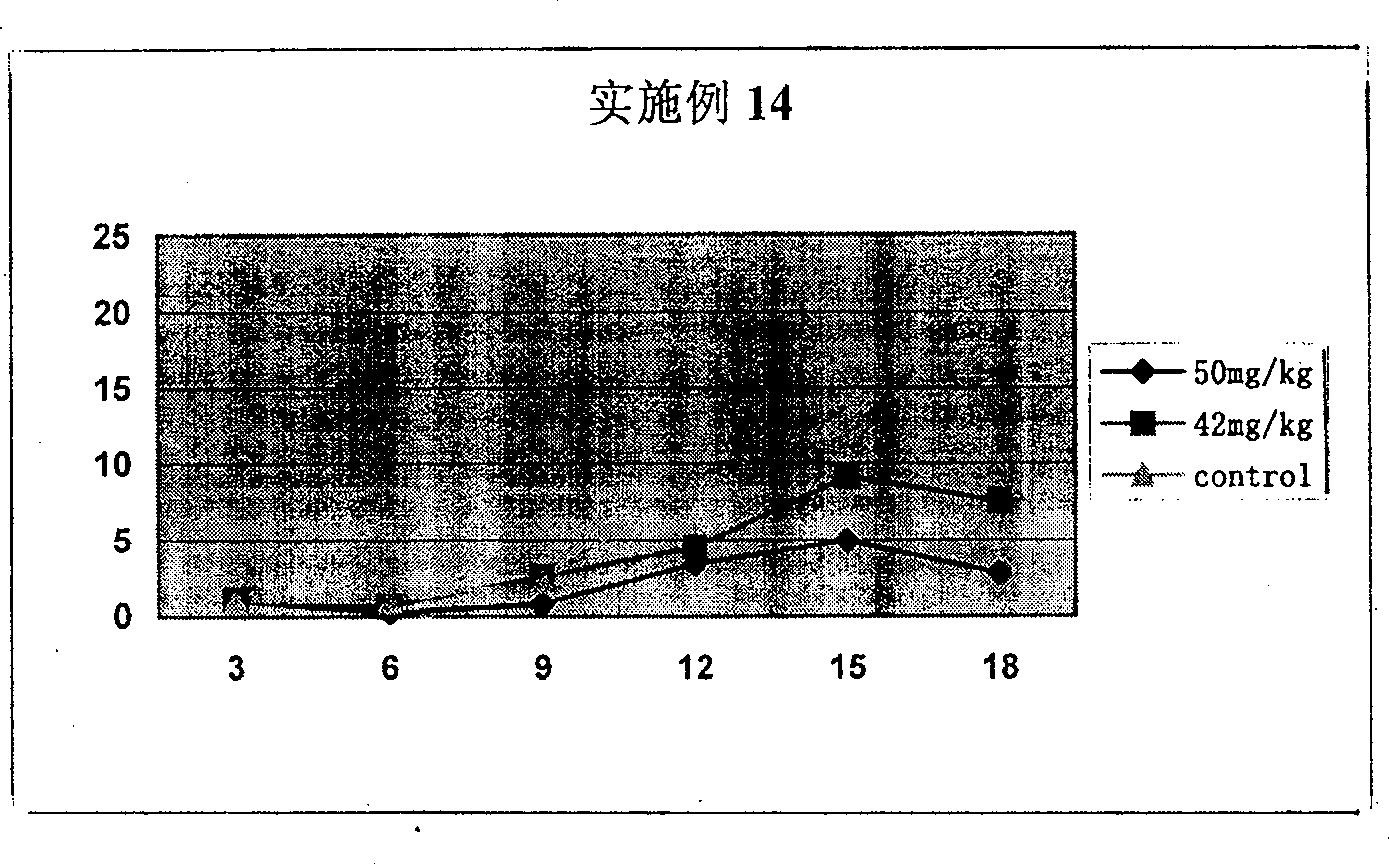
Bi-amido heterocyclic derivative with antitumour activity and its preparation method and use
A technology of anti-tumor activity and derivatives, applied in the fields of anti-tumor drugs, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve problems such as difficulties in bone marrow donors, inaccurate effects on patient survival chances, and high costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] p-Benzamidoacetophenone (IV)
[0069] 4-Aminoacetophenone (16.4g, 120mmol) and benzoyl chloride (14g, 100mmol) were dissolved in 1000ml of dichloromethane, triethylamine (18g, 180mmol) was added, stirred at room temperature for 4 hours, and the reaction solution was washed with 1N hydrochloric acid , dried over anhydrous sodium sulfate, and distilled off the solvent to obtain 20 g of the product, with a yield of 83.68%. m.p.126-128°C.
Embodiment 2
[0071] p-Butyrylaminoacetophenone (IV)
[0072] The method is the same as in Example 1 except that benzoyl chloride is replaced by butyryl chloride, and the yield is 71% in 17 g.
Embodiment 3
[0074] p-Ethoxybenzamidoacetophenone
[0075] The method is the same as in Example 1 except that benzoyl chloride is replaced with p-ethoxybenzoyl chloride, and the yield is 73% in 35 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com