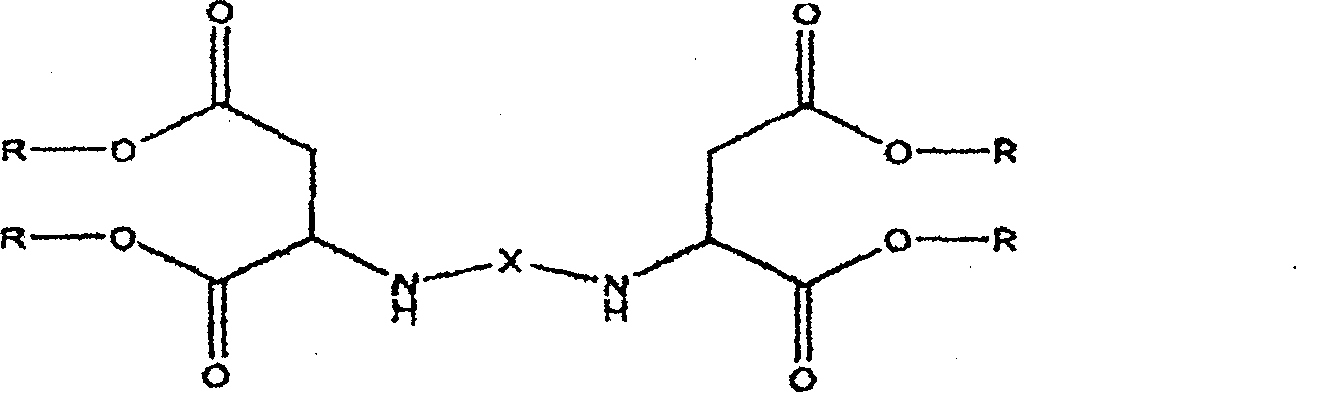
End-amido polyaspartic ester and method of manufacturing the same
A technology of polyaspartic acid ester and manufacturing method, applied in the field of amino-terminated polyaspartic acid ester and its manufacture, and manufacture of components for spraying polyurea type coatings, can solve the problem of polyurea materials without mentioning gel time Elongation at break physical properties and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
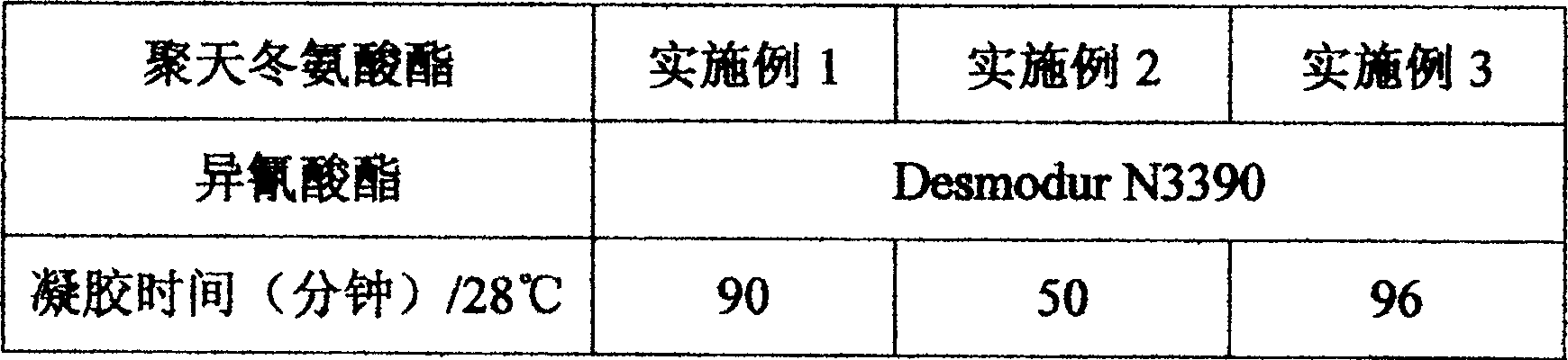
Embodiment 1
[0022] The three-necked flask was equipped with a stirrer, a heater, an addition funnel and a nitrogen inlet. 73.3 grams (0.700 equivalents) of 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane was put into the flask, and under the protection of nitrogen, it was added to the flask through the addition funnel within 1 hour. 446.9 grams (1.96 equivalents) of dibutyl maleate. Due to the exotherm of the reaction, the temperature of the reaction mixture rose to 40 °C, after which the temperature was maintained at 60 °C for 7 hours, at which time 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane had 100% conversion For polyaspartic acid ester. This mixture was added to a reaction bottle containing 144.9 grams of D-230 (1.26 equivalents) within 45 minutes, and the reaction was heated to 60°C for 12 hours, at which time 90% had been converted into polyaspartic acid ester, one month Afterwards, the reaction was 100% complete.
Embodiment 2
[0024]The three-necked flask was equipped with a stirrer, a heater, an addition funnel and a nitrogen inlet. 86.87 grams (0.730 equivalents) of 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane was put into the flask, and under the protection of nitrogen, it was added to the flask through the addition funnel within 1 hour. 499.3 grams (2.19 equivalents) of dibutyl maleate. Due to the exotherm of the reaction, the temperature of the reaction mixture rose to 40 °C, after which the temperature was maintained at 60 °C for 7 hours, at which time 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane had 100% conversion For polyaspartic acid ester. This mixture was added to a reaction bottle containing 229.9 grams of T-403 (1.46 equivalents) within 45 minutes, and the reaction was heated to 70°C for 12 hours, at which time 90% had been converted into polyaspartic acid ester, one month The post reaction was 100% complete.
Embodiment 3
[0026] The three-necked flask was equipped with a stirrer, a heater, an addition funnel and a nitrogen inlet. 90.57 grams (0.761 equivalents) of 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane was put into the flask, and under the protection of nitrogen, it was added to the flask through the addition funnel within 1 hour. 542.64 grams (2.38 equivalents) of dibutyl maleate. Due to the exotherm of the reaction, the temperature of the reaction mixture rose to 40 °C, after which the temperature was maintained at 60 °C for 7 hours, at which time 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane had 100% conversion For polyaspartic acid ester. This mixture was added to a reaction bottle containing 323.8 grams of D-400 (1.62 equivalents) within 45 minutes, and the reaction was heated to 70°C for 12 hours, at which time 90% had been converted into polyaspartic acid ester, one month The post reaction was 100% complete.
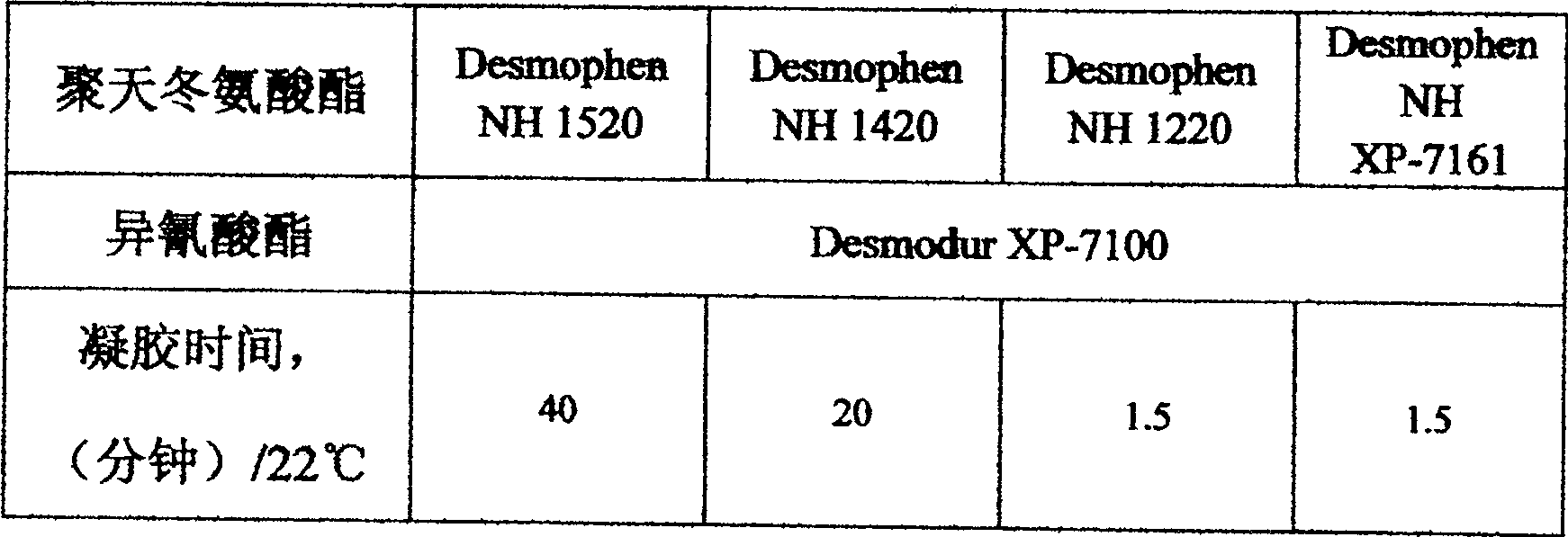
[0027] The reactivity presented by the polyaspartic acid ester obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com