Form board stationary beta hair clip ring analog and use in bacteriophage exhibition
A technology of immobilizing templates and mimetics, which is applied in the direction of microbial libraries, peptide preparation methods, chemical libraries, etc., can solve problems such as separation of affinity ligands, and achieve the effect of promoting strong activity and promoting binding-activity research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
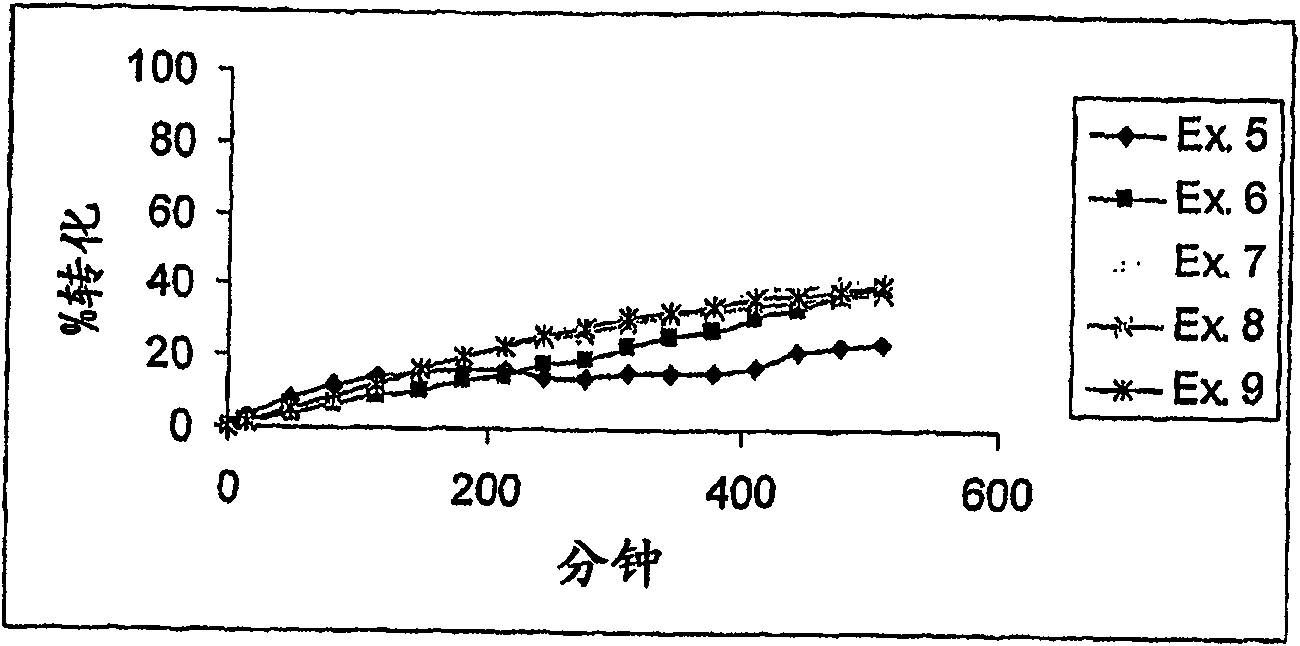
Examples
Embodiment 1
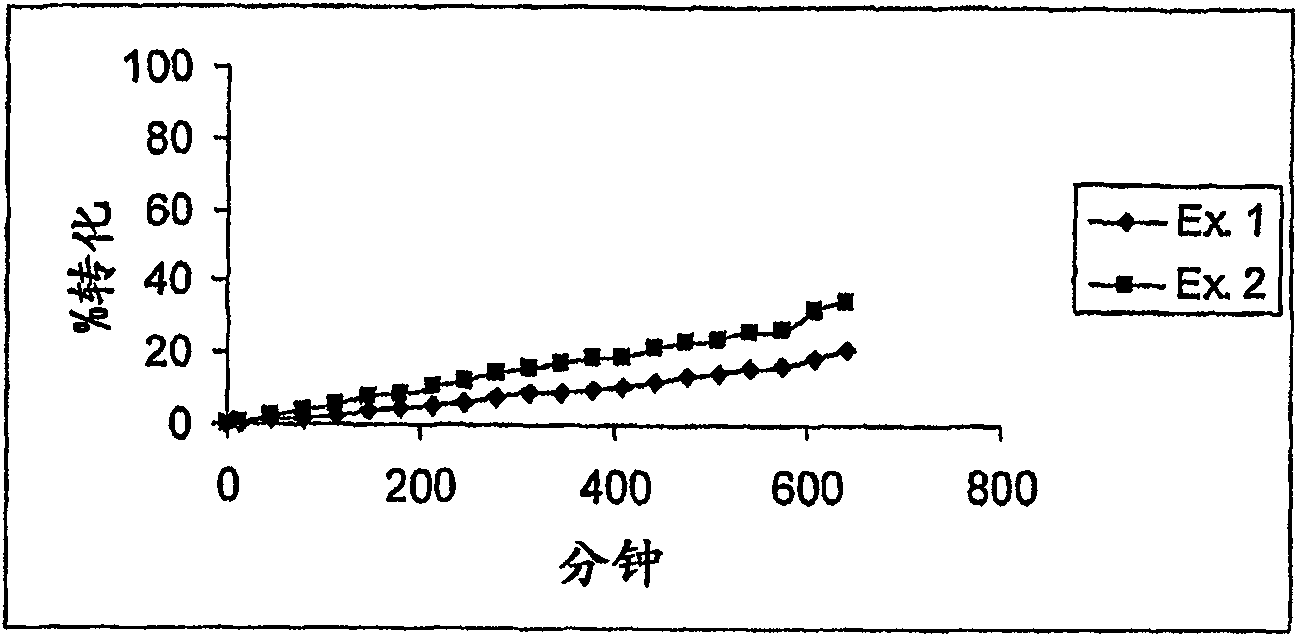
[0247] Example 1 (n=8) is shown in Table 1. Starting with the amino acid Cys, the peptide was synthesized, and the Cys was grafted onto the resin. The starting resin was Fmoc-Cys(Trt)-chlorotrityl resin (prepared as described above). Linear peptides were synthesized on solid supports according to Procedure 1 in the following order: Resin-Cys-P8-P7-P6-P5-P4-P3-P2-P1-Cys, followed by acylation, cleavage, deprotection as indicated , purification and cyclization. HPLC retention time (min) and mass determined using the above gradient: RT = 7.26 min, [M+H] + = 1281.3.
Embodiment 2
[0248] Example 2 (n=8) is shown in Table 1. Starting with the amino acid Lys, the peptide was synthesized, and Lys was grafted onto the resin. The starting resin was Fmoc-Lys(Boc)-chlorotrityl resin (prepared as above). Synthesize linear peptides on a solid support according to Procedure 1 in the following order: Resin-R2 -Cys-P8-P7-P6-P5-P4-P3-P2-P1-Cys-R 1 , followed by acylation, cleavage, deprotection, purification and cyclization of the peptide as indicated. HPLC retention time (min) and mass determined using the above gradient: RT = 6.41 min, [M+H] + = 871.4.
Embodiment 3
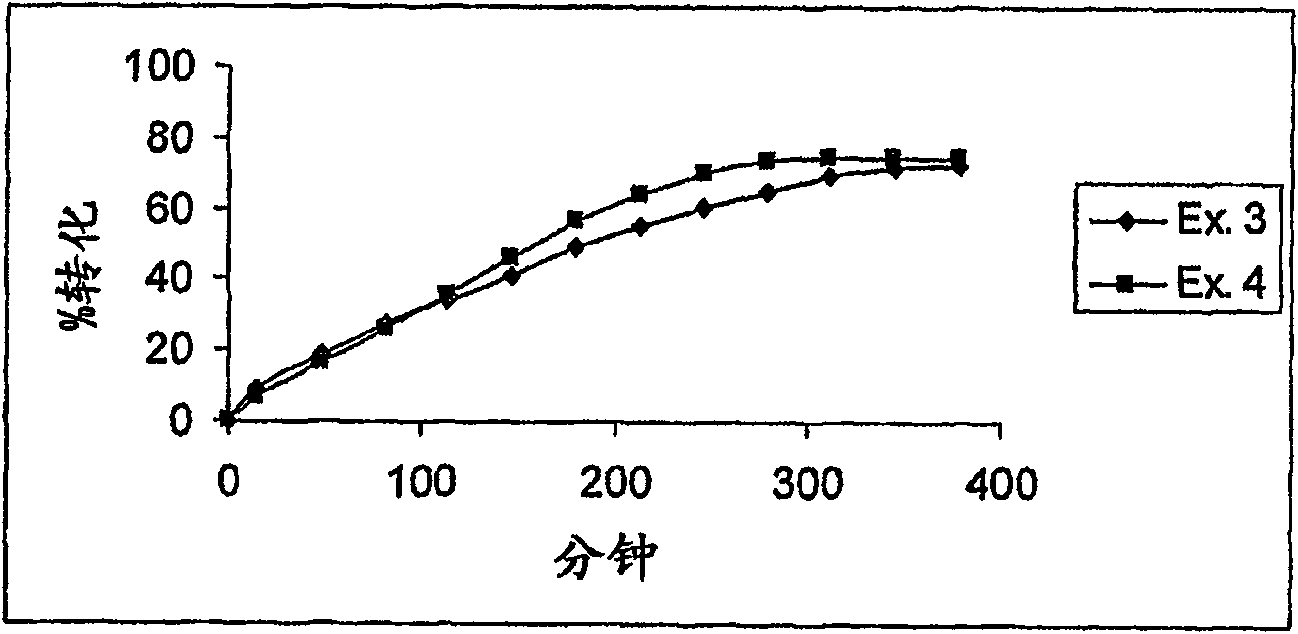
[0249] Example 3 (n=10) is shown in Table 2. Starting with the amino acid Cys, the peptide was synthesized, and the Cys was grafted onto the resin. The starting resin was Fmoc-Cys(Trt)-chlorotrityl resin (prepared as above). Synthesize linear peptides on a solid support according to Procedure 1 in the following order: Resin-Cys-P10-P9-P8-P7-P6-P5-P4-P3-P2-P1-Cys, and then acylate the peptides as indicated. Synthesis, fragmentation, deprotection, purification and cyclization. HPLC retention time (min) and mass determined using the above gradient: RT = 5.74 min, [M+H] + = 779.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com