HER3 antigen binding proteins binding to the beta-hairpin of HER3
A protein-binding technology, HVR-H2, is applied in the field of anti-HER3 antigen-binding protein, which can solve problems that have not yet been fully characterized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0596] 1. A method for selecting an antigen-binding protein that binds to human HER3 (and does not cross-react with human HER4), wherein the antigen-binding protein binds within the amino acid sequence PQPLVYNKLTFQLEPNPHT (SEQ ID NO: 1) of human HER3,
[0597] Which uses
[0598] a) at least one polypeptide comprising the amino acid sequence of SEQ ID NO: 1 selected from the group consisting of:
[0599]
[0600] and
[0601] b) at least one polypeptide comprising the amino acid sequence of SEQ ID NO:2 selected from the group consisting of:
[0602]
[0603] to select an antigen binding protein that exhibits binding to at least one polypeptide under a) and exhibits non-binding to at least one polypeptide under b),
[0604] And thus select an antigen-binding protein that binds within the amino acid sequence PQPLVYNKLTFQLEPNPHT (SEQ ID NO: 1) of human HER3 and does not cross-react with human HER4.
[0605] 2. The antigen-binding protein obtained by the selection method ...
Embodiment 1
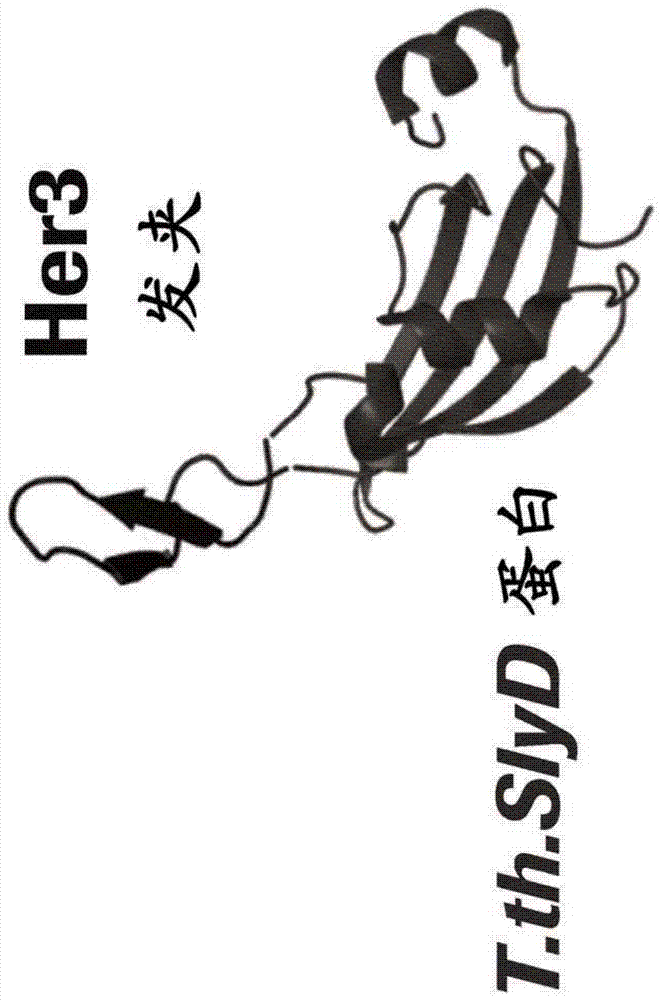
[0713] Antigen and Screening Protein Preparation - Generation of functional β-hairpin HER3 and β-hairpin HER4 constructs for selection of antibodies that bind HER3 β-hairpin and HER4 β-hairpin
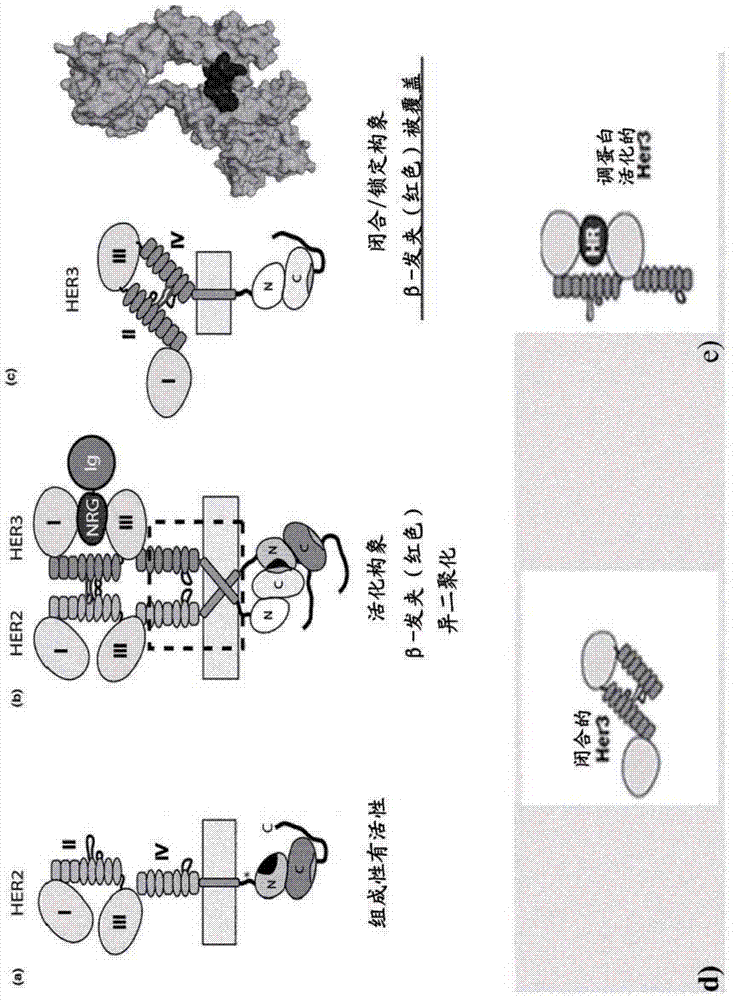
[0714] To generate functional β-hairpin HER3 and HER4 constructs, the amino acid sequences of the β-hairpins of HER3 (SEQ ID NO: 1 ) and HER4 (SEQ ID NO: 2) were grafted into the SlyD polypeptide framework containing the FKBP domain. In such constructs, the grafted β-hairpin is freely accessible, in contrast to the hidden structure in the native unactivated conformation of HER3 or HER4 (in the absence of a ligand such as HRG) (see figure 1 c and 1d, where the β-hairpin of HER3 is hidden).
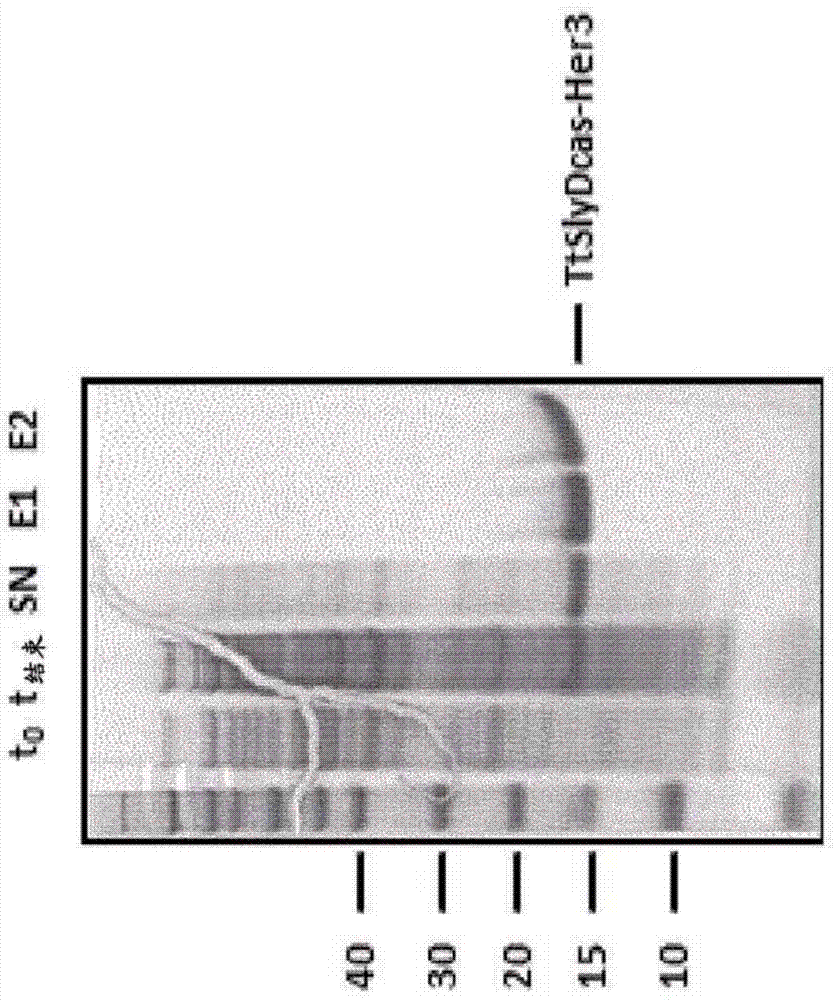
[0715]All fusion SlyD polypeptides can be purified and refolded using almost the same protocol. Escherichia coli BL21(DE3) cells transformed with specific expression plasmids were grown at 37°C in LB medium containing corresponding antibiotics for selective growth (kanamycin 30 μg / ml, or ampicillin...
Embodiment 2
[0725] a) Immunization and selection of HER3 antibody
[0726] To generate antibodies against the HER3 β-hairpin, Balb / C, NMRI or SJL mice were immunized with different antigens. The following proteins were used as antigens: full-length HER3 ECD, or epitope scaffold proteins TtSlyD-FKBP12-Her3, TtSlyDcys-Her3, TtSlyDcas-Her3, TgSlyDcys-Her3 and TgSlyDser-Her3. The TtSlyD-FKBP12-Her3 variant represents the first epitope scaffold for generating HER3 dimerization domain-specific antibodies. Although the general rationale for using SlyD variants as epitope scaffolds could have been demonstrated earlier using the first generation SlyD-FKBP12 scaffolds, improved scaffold variants with higher stability were developed. These SlyD variants were derived from Thermus thermophilus and Thermus gamma-resistant.
[0727] All mice were immunized three times at time points 0, 6 and 10 weeks after the start of the immunization campaign. At each time point each mouse was immunized with 100 μg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com