Uses of spatial configuration to modulate protein function
A technology of interferon and compound interferon, which is applied in the direction of medical preparations containing active ingredients, nebulizers for treatment, peptide/protein components, etc., can solve the problem of not being able to inhibit e-antigen and s-antigen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
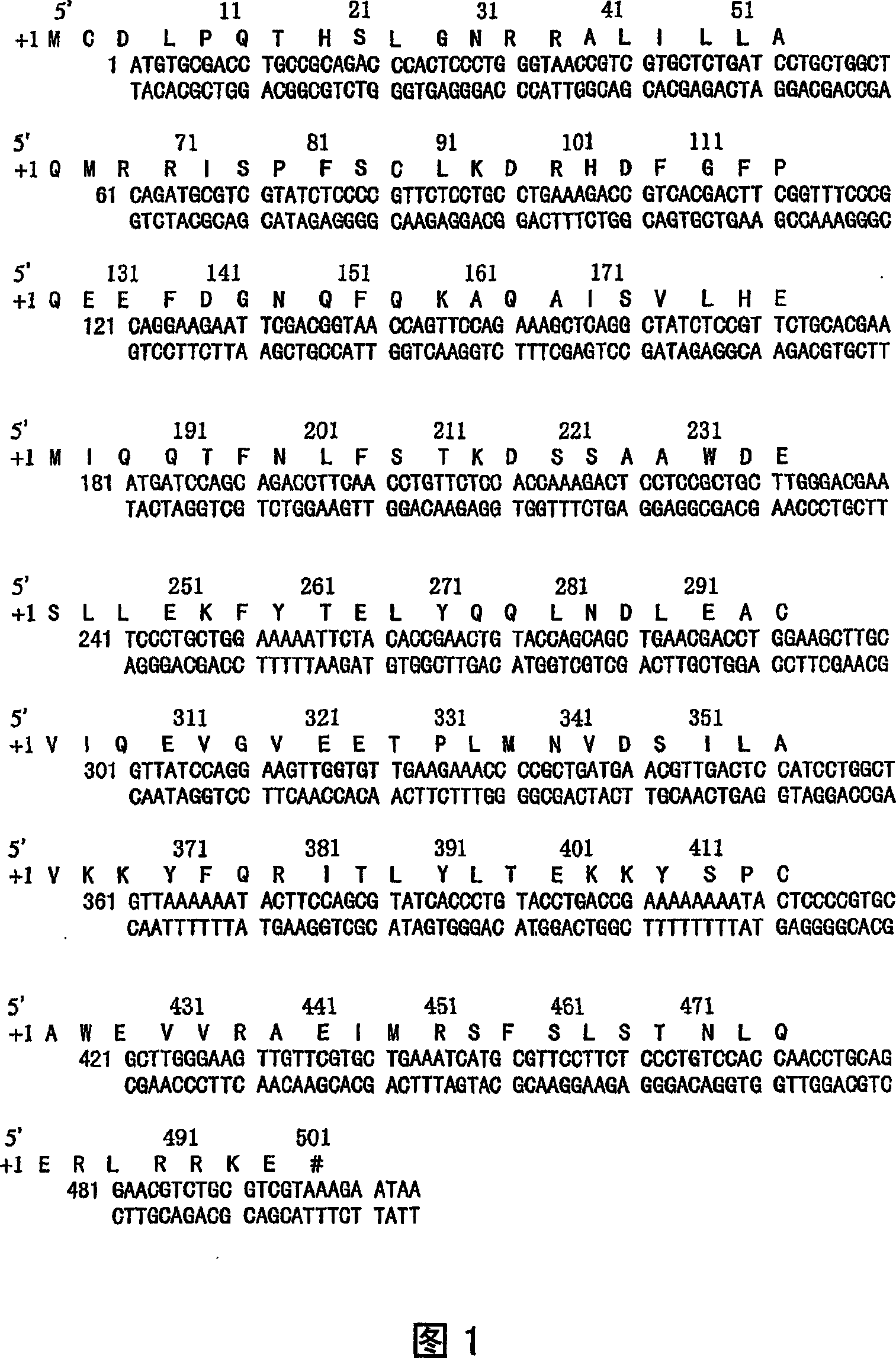
[0152] Recombinant high-efficiency composite interferon (rSIFN-co) is a new interferon molecule constructed by genetic engineering from the most common conserved amino acids in several natural human alpha interferon subtypes. It has been proved that rSIFN-co has broad-spectrum interferon activity, such as strong antiviral and tumor suppressive activity, especially in anti-chronic hepatitis C.
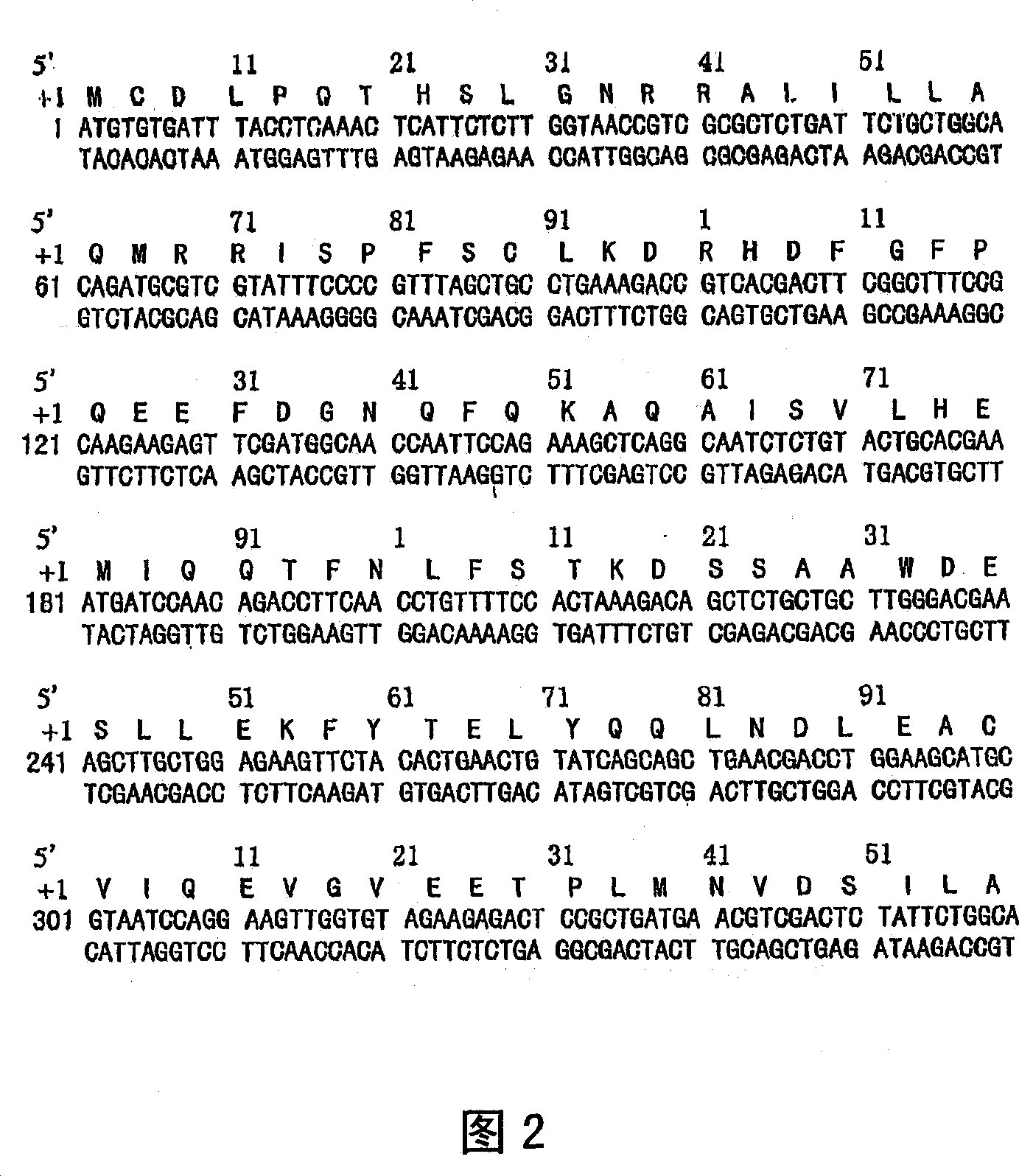
[0153] According to the published rSIFN-co coding DNA sequence and deduced amino acid sequence (Fig. 1), we redesigned the cDNA coding sequence of rSIFN-co using the preferentially expressed codons in Escherichia coli, and then artificially synthesized the full-length cDNA gene of rSIFN-co.
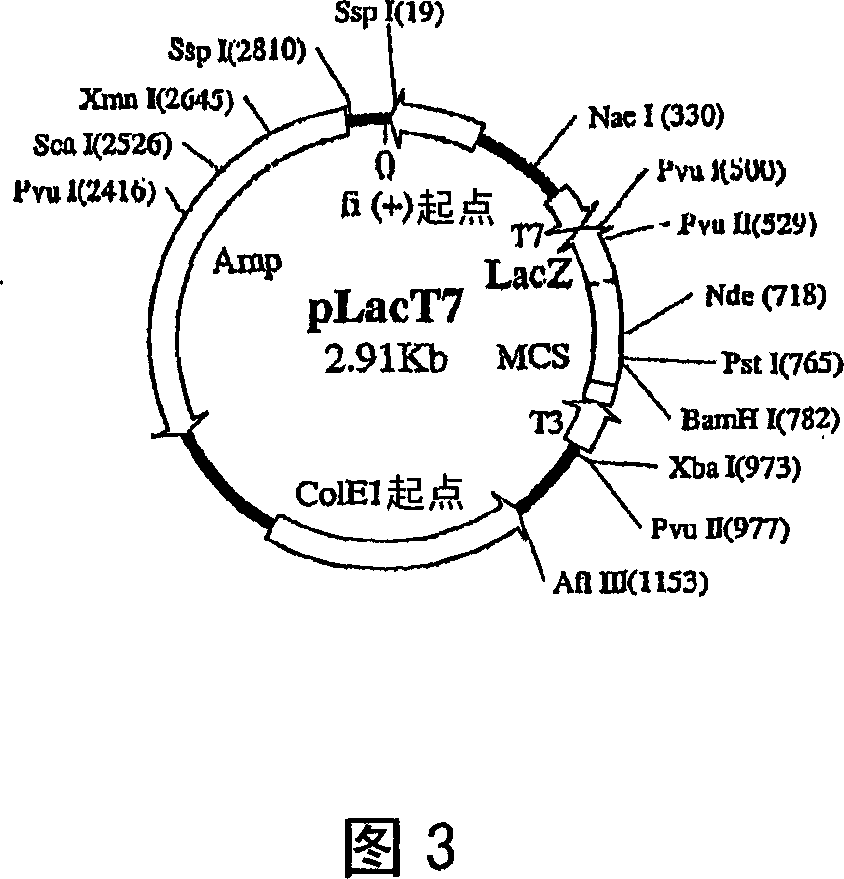
[0154] In order to obtain high-purity rSIFN-co protein, the full-length cDNA sequence of rSIFN-co was cloned into the high-efficiency expression vector of Escherichia coli, and then the strong P in the vector was induced and activated by L-arabinose BAD The promoter mediated the high expression of ...
Embodiment 2
[0263] Isolation and purification of rSIFN-co
[0264] 1. Fermentation
[0265] Inoculate the recombinant strains in LB medium and culture overnight (about 18 hours) at 37°C in shake flasks (200rpm), add 30% glycerol to the fermentation broth to a final concentration of 15%, divide into 1ml each, and store at -20°C Preserved as a production strain.
[0266] The production strain was added to LB medium at a ratio of 1%, and the scale of the strain was expanded overnight by culturing in a shake flask (200 rpm) at 37°C. Add 10% into RM medium and culture at 37°C. Ferment to OD 600 To reach 2.0 or so, add arabinose (20%) to a final concentration of 0.02% for induction. Stop culturing after 4 hours, centrifuge, and collect bacteria. The bacterial cell pellet was resuspended with an appropriate amount of buffer A, placed at -20°C to pass through the liquid, taken out and melted, destructed with a homogenizer, centrifuged, washed with buffers B and C separately, and washed once ...
Embodiment 3
[0331] Stability of recombinant high-efficiency compound interferon freeze-dried powder for injection
[0332] We conducted a stability test on three batches of samples of the recombinant high-efficiency compound interferon freeze-dried powder, each with two specifications. The test start time: April 2000.
[0333] 1. Sample source
[0334] The samples were provided by Sichuan Huiyang Life Engineering Co., Ltd., and the batch numbers are: 990101-03, 990101-05, 990102-03, 990102-05, 990103-03, 990103-05.
[0335] 2. Sample standard
[0336] Each sample in this experiment should meet the requirements in the table below before the test.
[0337] Table 1 Test sample standard
[0338] indicators
standard
1. Appearance
white puffy body
2. Dissolving time
Water for injection dissolves rapidly at room temperature (within 2 minutes)
3. Clarity
Colorless or slightly opalescent clear liquid, should not be turbid, contain foreign mat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com