Nicotinic receptor agonists for the treatment of inflammatory diseases
A technology for inflammatory diseases and nicotinic receptors, which is applied in some continuation applications and can solve problems such as low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0348] In Vivo HP Study-I-Hypersensitivity-Like Inflammation
[0349] Effects of nicotinic agonists on chronically induced hypersensitivity pneumonitis (HP) in mice.
[0350] Stimulation of nicotinic receptors with nicotine was demonstrated to down-regulate immune responses to HP antigens through inhibition of inflammatory cytokines and inhibition of specific antigen-mediated cell activation.
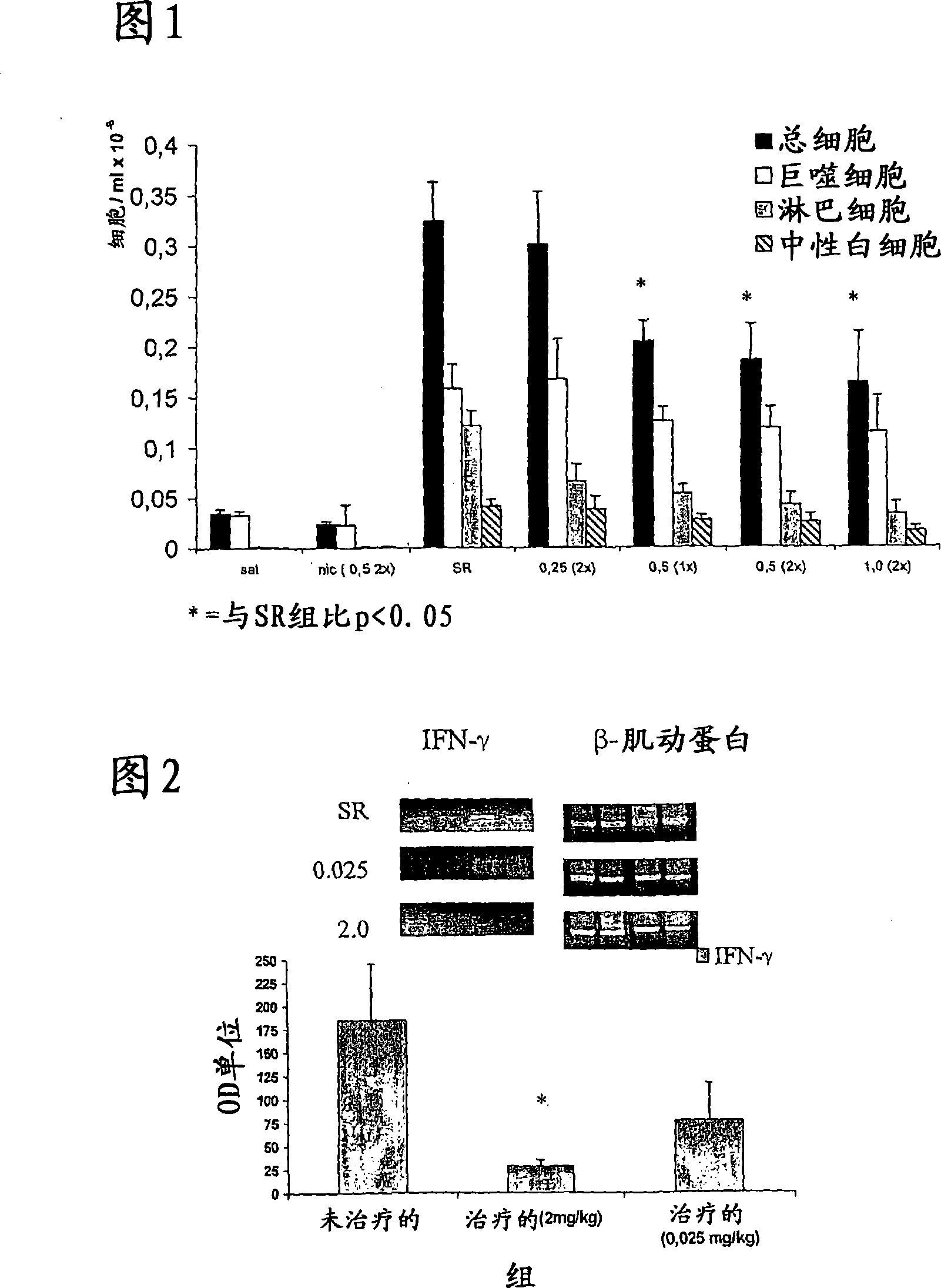
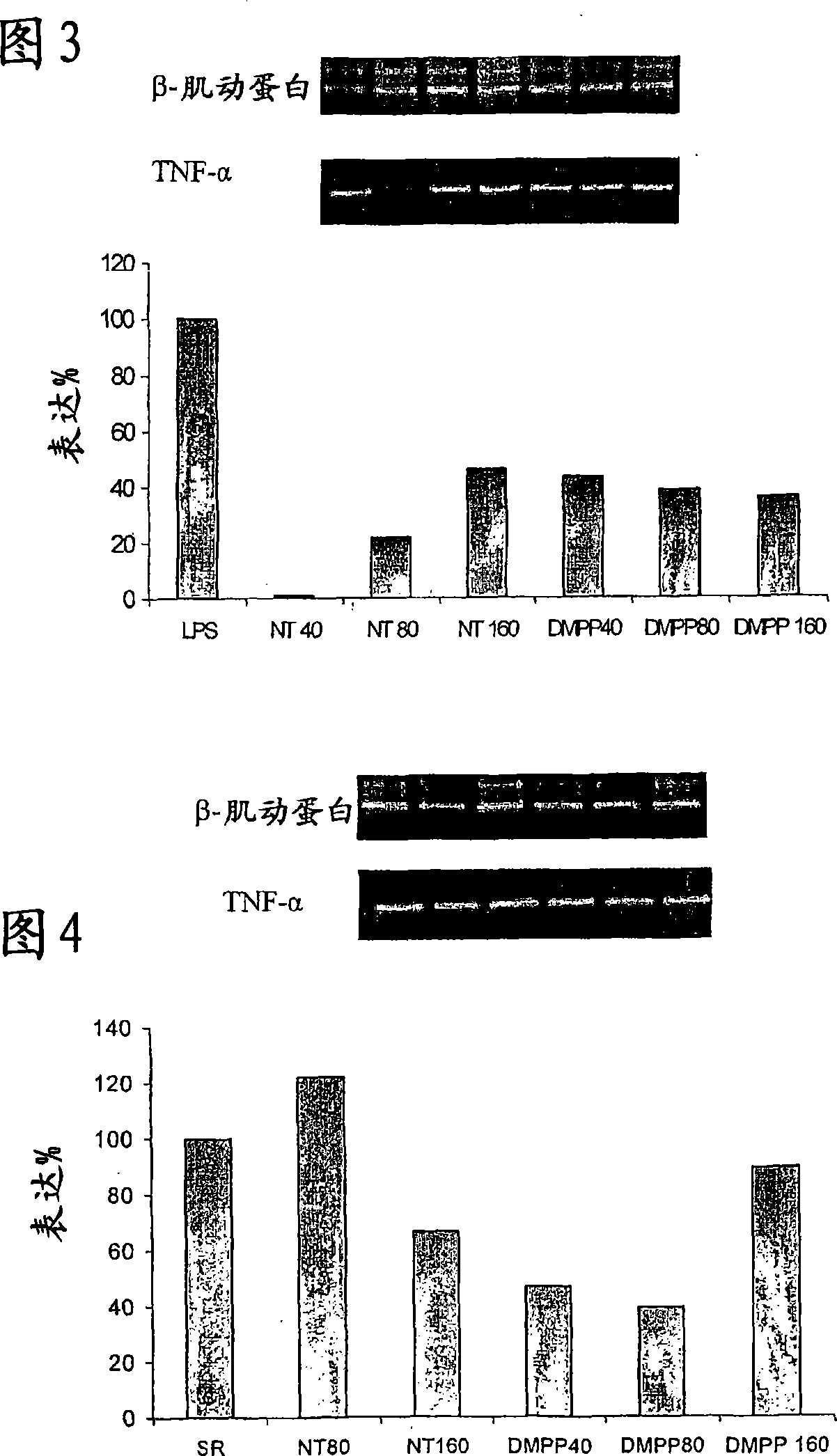
[0351] As mentioned above, this model was chosen because the incidence of HP is lower in smokers than in nonsmokers (50), and because the model is well described. HP was induced by administration of an HP form of farmer's lung pathogen Saccharopolyspora straight comma (SR) antigen (51). Mice were also treated with intraperitoneal (IP) nicotine at doses ranging from 0.5-2.0 mg / kg twice a day. Nicotine administration significantly reduced the overall cell numbers found in the bronchoalveolar lavage (BAL) of these mice. The population most affected by nicotine treatment is lymphocytes, ...
Embodiment II
[0353] In vitro studies demonstrating the effect of nicotinic agonists on cytokine expression.
[0354] To further elucidate the mechanisms involved in the inhibitory effect of nicotine on an in vivo model, an alveolar macrophage cell line was used.
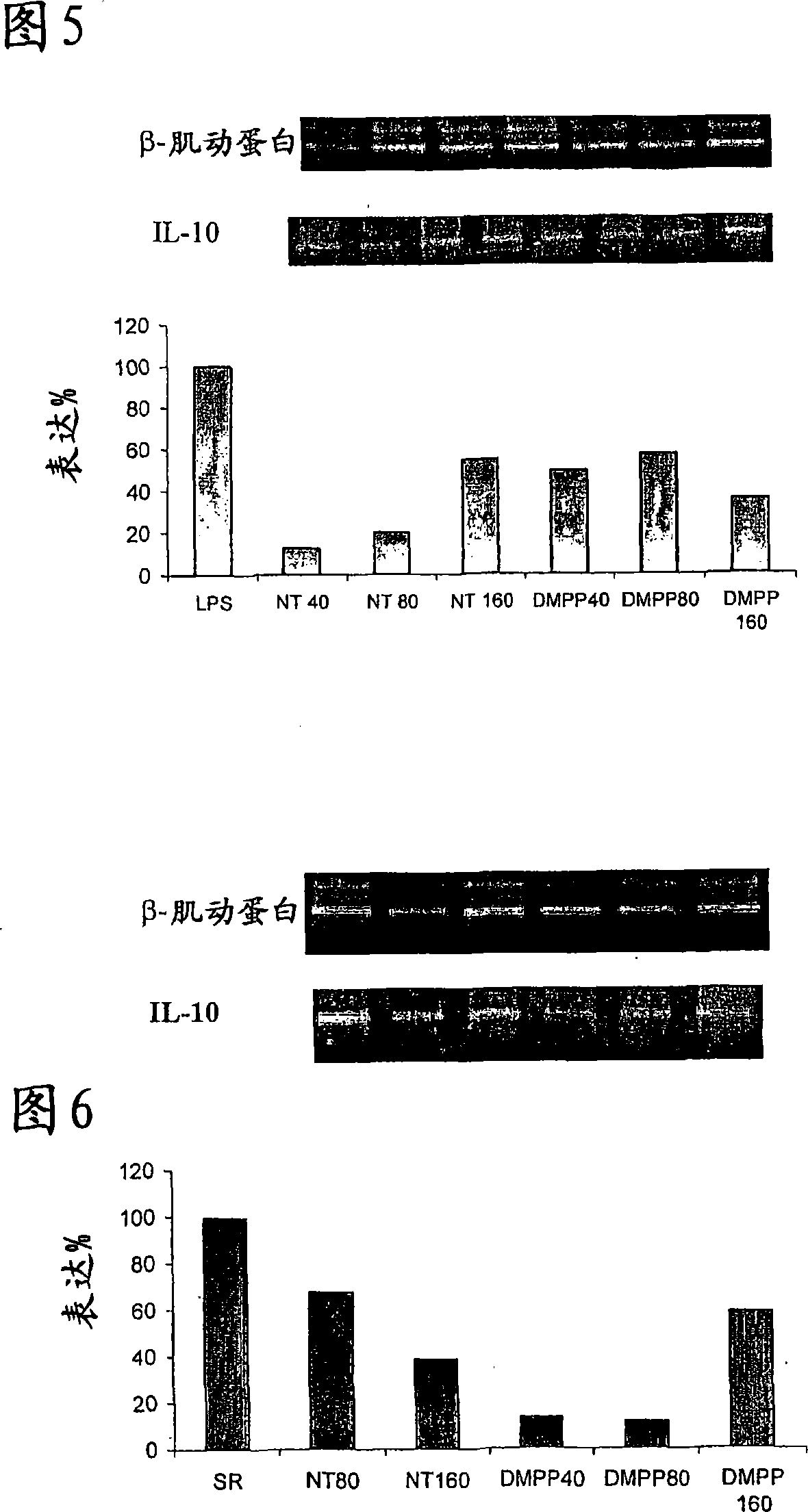
[0355] The effect of nicotine or DMPP treatment on TNF-α, IL-10 mRNA expression on AMJ2-C11 cells was tested by RT-PCR. These cytokines are involved in the development of pulmonary inflammatory diseases such as HP, asthma and sarcoidosis (52-55). Nicotine and DMPP treatment showed a significant decrease in TNF mRNA expression (up to 98% decrease in LPS-stimulated cells treated with 40 [mu]M nicotine), but not in a dose-dependent manner. Referring to Figure 3, where results are expressed as % expression, 100% is assigned to the LPS group alone. Band intensities were obtained by dividing the TNF-α band intensity by the β-actin band intensity. Treatment of stimulated cells with different doses (40-160 μM, nicotine and DMPP) induc...
Embodiment III
[0358] In vitro effects of nicotinic agonists on the expression of co-stimulatory molecules.
[0359] The effect of nicotine and DMPP on B7 (CD80) molecule expression was tested in vitro. AMJ2-C11 cells (mouse alveolar macrophages from ATCC) were incubated with 40 μM nicotine or DMPP and stimulated with LPS (0.1 μg / ml) or SR (50 μg / ml) for 48 hours. The percentage of CD80 expression in treated cells was approximately half of that measured in LPS- and SR-stimulated untreated cells. Referring to Figure 8(a), it was shown that nicotine treatment (4 [mu]M, 48 hours) reduced the expression in LPS-stimulated cells to 20%. Referring again to Fig. 8(b), it was shown that DMPP treatment (40 [mu]M, 48 hours) reduced the expression in LPS-stimulated cells to 17% and in SR-stimulated cells to 20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com