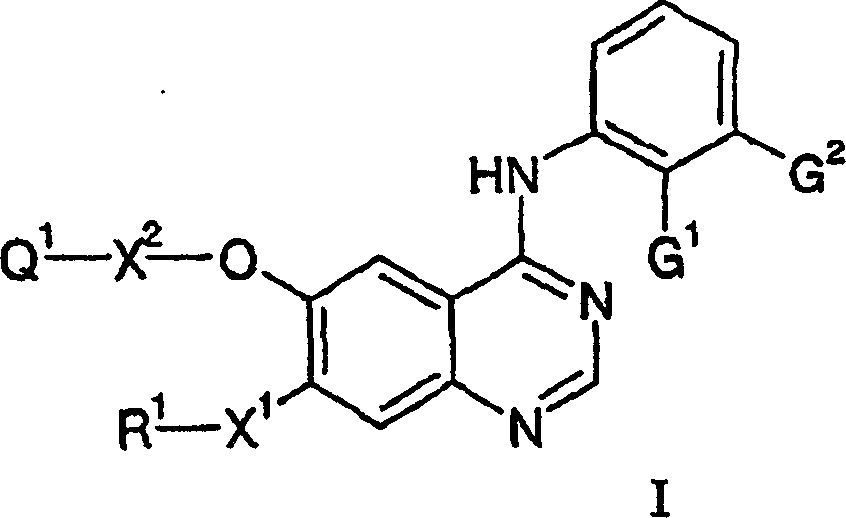
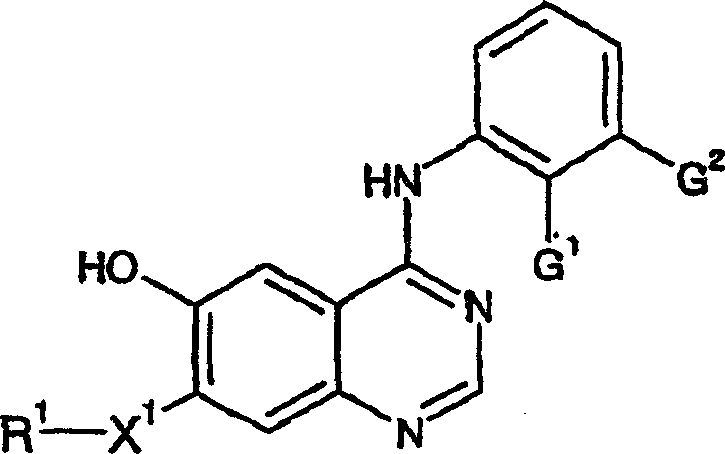
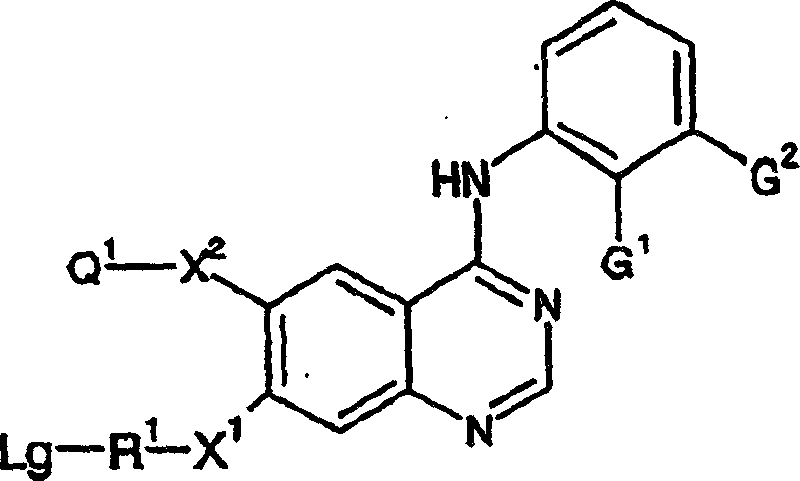
4-anilino quinazoline derivatives as antiproliferative agents
A kind of derivative, the technology of quinazoline, applied in the medicine of solid tumor disease, the field of preparation of described quinazoline derivatives, can solve the problems such as unpublished
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0861] 4-(3-Chloro-2-fluoroanilino)-7-methoxy-6-[(1-methylpyrrolidin-3-yl)oxy]quinazoline
[0862]
[0863] 1- Methyl-3-pyrrolidinol (658 μl, 6.0 mmol) and triphenylphosphine (1572 mg, 6.0 mmol). The suspension was cooled to below 0°C under nitrogen atmosphere. A solution of di-tert-butyl azodicarboxylate (1380 mg, 6 mmol) in DCM (20 ml) was added dropwise over 15 minutes. The resulting light brown solution was warmed to room temperature and stirred overnight. The solution was evaporated and the residue was purified by chromatography eluting with 0-5% methanol in DCM. Appropriate fractions were combined, evaporated and the crude product (230mg) redissolved in 1:1 methanol / DCM (5ml). Etherified HCl (1M, 1.14ml) was added and the mixture was evaporated. Crystallization from methanol / ether gave the title product as the hydrochloride salt as a white crystalline solid (154 mg, 16%);
[0864] 1 H NMR (hydrochloride):
[0865] ...
Embodiment 2
[0868] 4-(3-Chloro-2-fluoroanilino)-7-methoxy-6-[(piperidin-4-yl)oxy]quinazoline
[0869]
[0870] With 6-{[(1-tert-butoxycarbonyl)piperidin-4-yl]oxyl}-4-(3-chloro-2-fluoroanilino)-7-methoxyquinazoline (reference implementation Example 3; 350mg, 0.70mmol) was dissolved in trifluoroacetic acid (5ml) and the solution was left for 2 hours. Excess trifluoroacetic acid was evaporated and the residue was azeotroped twice with DCM. The residue was purified by chromatography with 0-4% (7:1 MeOH / conc. NH 4 OH aq) in DCM for elution. Evaporation of the appropriate fractions gave the product as an off-white solid (270 mg, 96%);
[0871] 1 H NMR: 1.53-1.64 (m, 2H), 2.00-2.05 (m, 2H), 2.64-2.72 (m, 2H), 3.00-3.07 (m, 2H), 3.92 (s, 3H), 4.60 (m, 1H), 7.20(s, 1H), 7.26(ddd, 1H), 7.47(dd, 1H), 7.50(dd, 1H), 7.82(s, 1H), 8.34(s, 1H), 9.56(s, 1H );
[0872] mass spectrometry : 403.2, 405.2.
Embodiment 3
[0874] 4-(3-Chloro-2-fluoroanilino)-7-methoxy-6-[(piperidin-4-yl)methoxy]quinazoline
[0875]
[0876] Using 6-{[(1-tert-butoxycarbonyl)piperidin-4-yl]methoxy}-4-(3-chloro-2-fluoroanilino)-7-methoxyquinazoline (reference Example 4) The procedure described in Example 2 was repeated. The title compound was obtained in 91% yield;
[0877] 1 H NMR: 1.45-1.61 (m, 2H), 1.95-2.00 (m, 2H), 2.18 (m, 1H), 2.92 (m, 2H). 3.25-3.35 (m, 2H), 3.93 (s, 3H) , 4.03(d, 2H), 7.20(s, 1H), 7.26(dd, 1H), 7.46(dd, 1H), 7.50(dd, 1H), 7.89(s, 1H), 8.36(s, 1H), 8.72(br.s, 1H), 9.74(s, 1H);
[0878] mass spectrometry : 417.4, 419.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com