Optically enhanced chiral ionic liquids
A technology for ionic liquids and optical isomers, applied in the field of n.200-01 and methods, which can solve problems such as asymmetric results
Inactive Publication Date: 2007-09-12
SIGMA ALDRICH CO LLC
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The result is asymmetrical
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
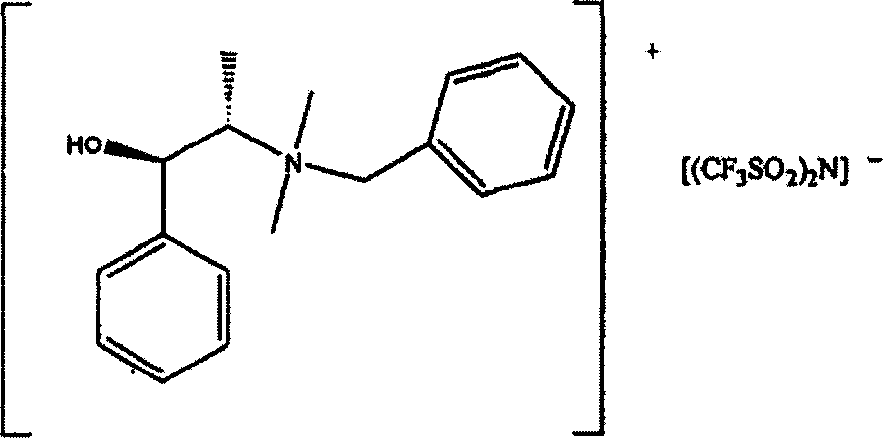
[0172] When N,N-dimethylephedrine NTf2 was used as a cosolvent, the reaction gave about 3% enantiomeric excess (e.e). The reaction was carried out by NaBH at room temperature 4 Reduction of methyl phenyl ketones. Pass GC, use 20m Chiraldex TM G-PN column analysis of the reaction mixture.
[0173]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
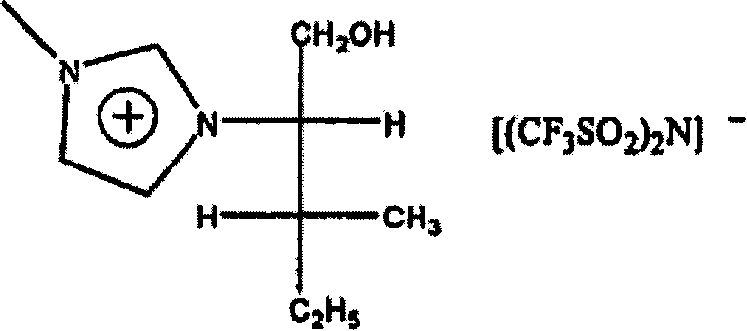
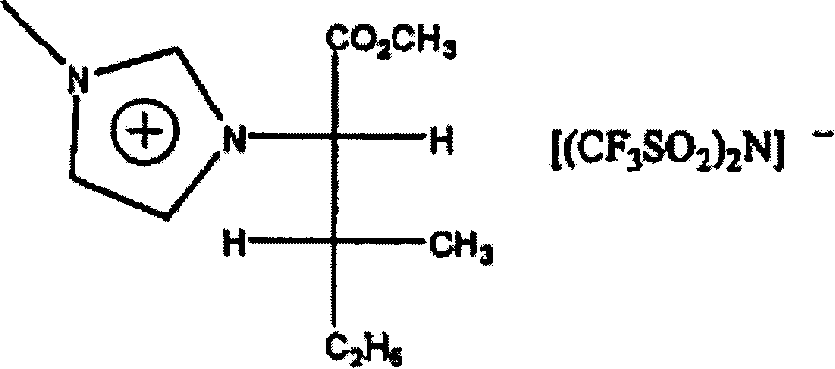
The invention relates to the use of optically enhanced chiral ionic liquids, particularly for gas chromatography and as a reaction solvent. Specific optically enhanced chiral cationic liquids are described as is a class of optically enhanced chiral anionic liquids.
Description
[0001] Cross References to Related Applications [0002] This application claims priority to US Provisional Patent Application No. 60 / 586,782, filed July 9, 2004, the contents of which are hereby incorporated by reference. Background of the invention [0003] Achiral ionic liquids have been used as solvents in the past. Racemic chiral liquids are also used as solvents. See Yasuhiro Ishida et al., "Design and synthesis of a novel imidazolium-basedionic liquid with planar chirality," Chem. Commun. 2240-41 (2002) (Copyright by the Royal Society of Chemistry). Solvents for enantiomerically increased chiral cations are also described. See Wasserscheid et al., "Synthesis and properties of ionic liquids derived from the 'chiral pool'," Chem. Commun. 200-01 (2002) and Weiliang Bao et al., "Synthesis of Chiral Ionic Liquids from Natural Amino Acids," 68 J.Org . Chem. 591 (2003). These solvents are not described as a medium for carrying out the subsequent reactio...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/18C07C307/02
CPCC07C67/333C07C311/48C07B2200/07C07D213/18C07C231/20C07C2101/14C07C2101/02C07C2103/90C07C309/65C07C29/143C07C2601/02C07C2601/14C07C2603/90C07C69/753C07C233/18C07C33/22C07C35/08C07C35/21C07C33/34
Inventor D·W·阿姆斯特朗J·丁
Owner SIGMA ALDRICH CO LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com