Immunotherapy of autoimmune disorders
An autoimmune disease, selected from the technology, applied in the field of producing the conjugate, cytotoxic drug/B cell depleting agent conjugate, B cell depleting agent conjugate, combined therapy, can solve the inability of autoimmune disease Effectively treat issues such as autoimmune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
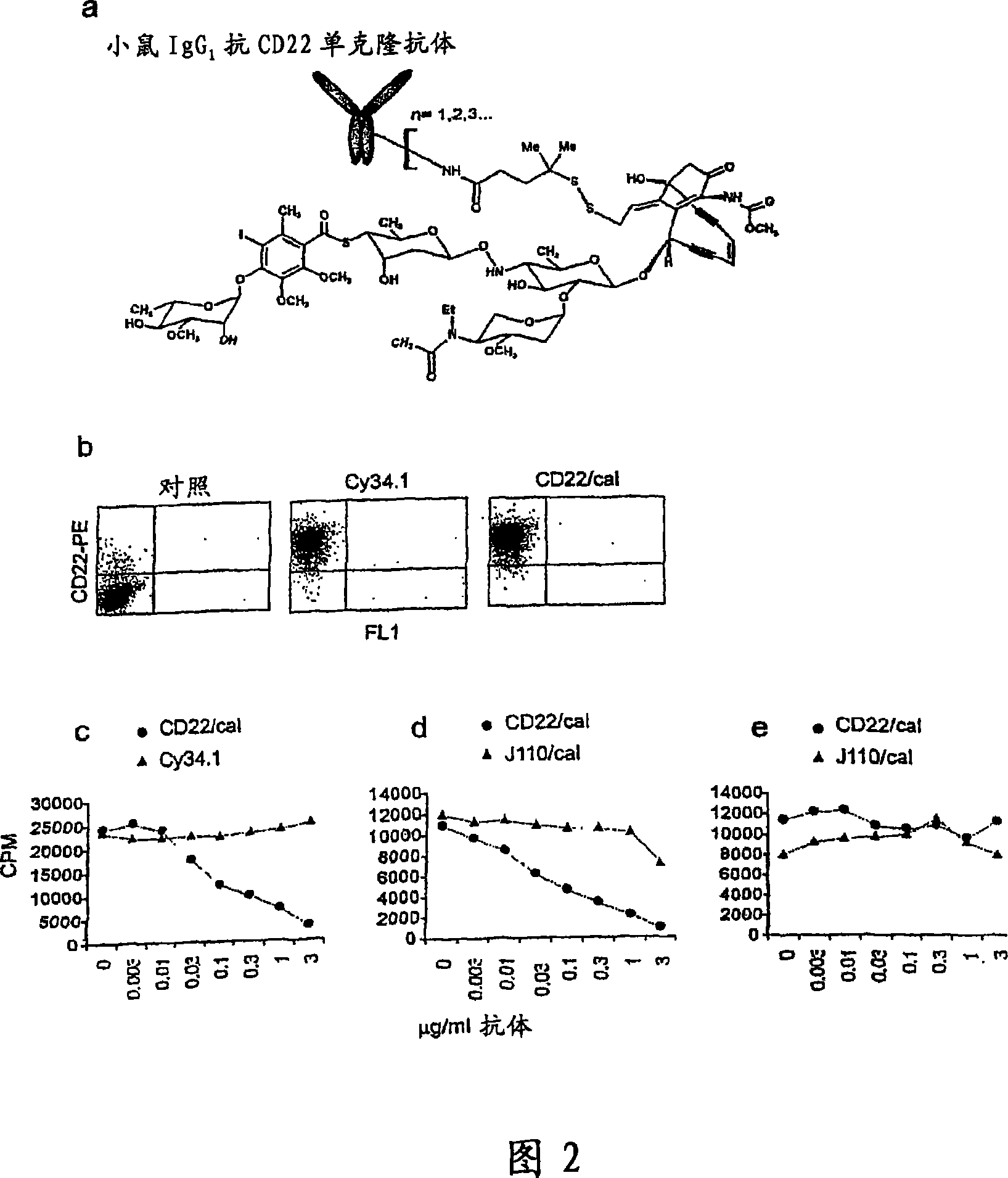
[0317] B cell depletion and anti-CD22 / calicheamicin immunoconjugate inhibit collagen-induced arthritis in a C57BL / 6 mouse model.
[0318]The study was carried out to test the role of B cell depletion in a mouse model of rheumatoid arthritis. The B cell depleting compound used in this study was mouse anti-CD22 mAb (Cy34.12) conjugated to calicheamicin, a member of the enediyne class of antitumor antibiotics ("conjugate").
[0319] Mice on a C57BL / 6 background were used due to Cy34.12 reactivity. In the in vitro cytotoxicity assay, purified primary B cells from male C57BL / 6 mice were cultured with the conjugate, and their proliferation under LPS stimulation was studied 48 hours after the initiation of culture.
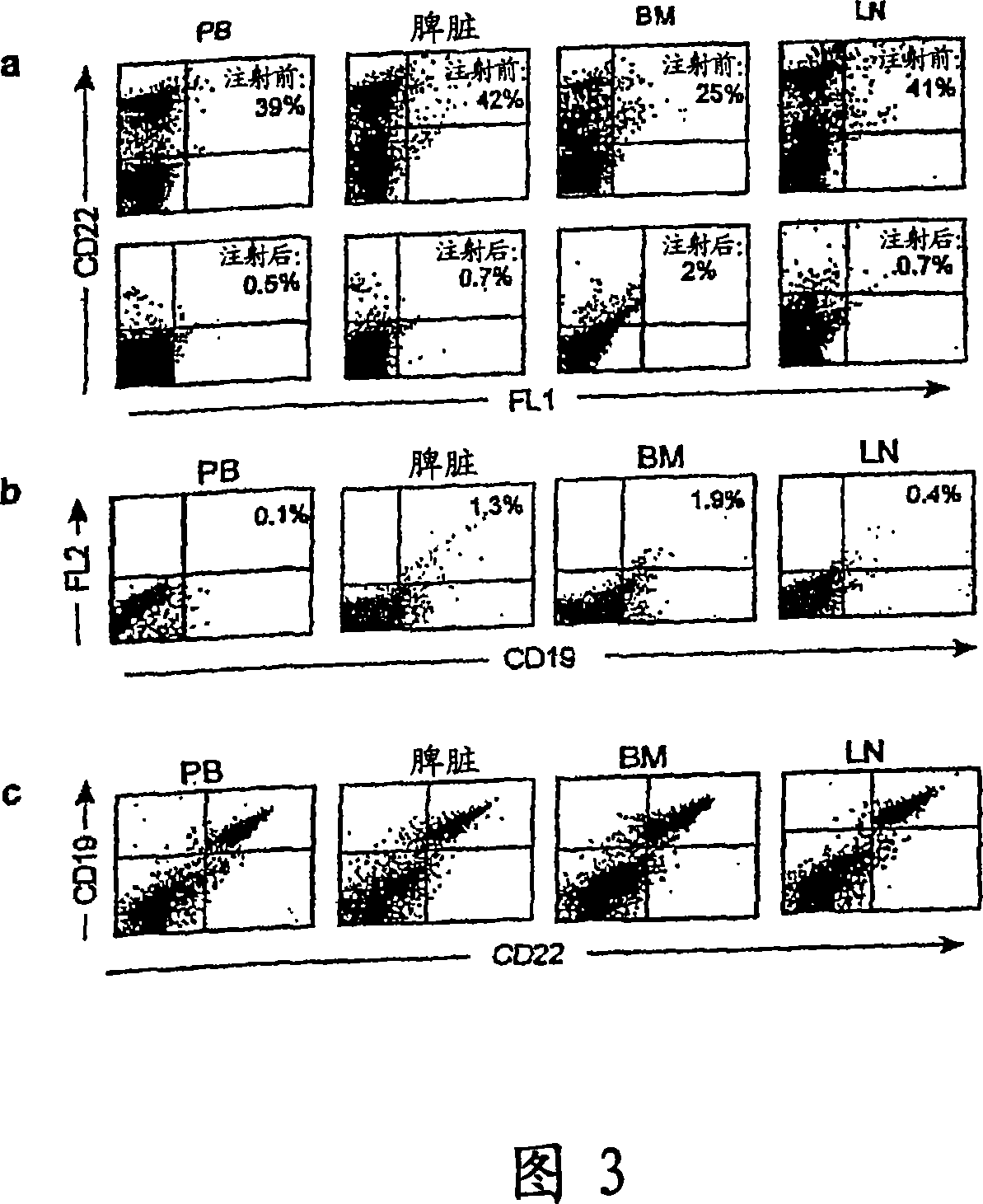
[0320] In the in vivo cytotoxicity studies, male C57BL / 6 mice were injected intraperitoneally (i.p.) twice (day 0 and day 5) with the conjugate at a dose of 160 pg / kg / injection of calicheamicin. B cell depletion was monitored by flow cytometry in serial samples of bone...
Embodiment 2
[0324] Depletion of B cells targeting CD22 suppresses clinical and histological arthritis in a collagen-induced arthritis (CIA) model
[0325] This study was carried out to test the role of B cell depletion in a collagen-induced arthritis (CIA) model. The B cell depleting compound used in this study (referred to herein as CD22 / cal) is a conjugate of an anti-mouse CD22 monoclonal antibody (mAb) and N-acetyl-γ-calicheamicin dimethyl acid, belonging to the A member of the enediyne class of antitumor antibiotics. Anti-mouse CD22 is a mouse IgGl mAb purified from a Cy34.1 hybridoma (American Type Culture Collection, Rockville, MD). The synthesis of antibody / calicheamicin conjugates was previously described (Hamann, P.R. et al., An anti-CD33 antibody / calicheamicin conjugate for treatment of acute myeloid leukemia. Choice of linker. Bioconjug Chem 13, 40-6 (2002)). The average CD22 / cal loading was 17 to 30 μg calicheamicin / mg antibody protein (1.2-2.6 moles calicheamicin / mole antib...
Embodiment 3
[0358] Effects of CD22 / cal-induced B cell depletion on clinical scores and antibody responses in a collagen-induced (CIA) model
[0359] B cell depletion in the CIA model
[0360] Complete Freund's adjuvant (CFA) (DifcoLaboratory, Detroit, MI) (containing 5 mg / ml inactivated Mycobacterium tuberculosis (H37Ra) 33 ) with 100 μg bovine type II collagen (CII) (Chondrex, Redmond, WA) subdermally immunized once (day 0) to induce CIA. CII-immunized mice were intraperitoneally injected twice (day 5 and day 10) with CD22 / cal or control GG5 / cal (160 μg / kg / injection). The paws were evaluated for clinical arthritis, and each paw was scored on a 4-point scale: 0, normal paw; 1, minimal swelling or redness; 2, redness and swelling of the entire front paw; 3, whole paw Redness and swelling of extremities; 4. Joint deformation and / or joint ankylosis.
[0361] Anti-Type II Collagen Antibody ELISA
[0362] The level of anti-II collagen IgG2b antibody was detected by standard ELISA method, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com