Prepn of specific antibody of provera acetate and method of using the antibody in homogenous or heterogenous enzyme-linked immune analysis
A technology of medroxyprogesterone acetate and chlormadinone acetate, applied in the field of establishment of ELISA analysis methods, can solve the problems of less research on ELISA methods, and achieve the effect of high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
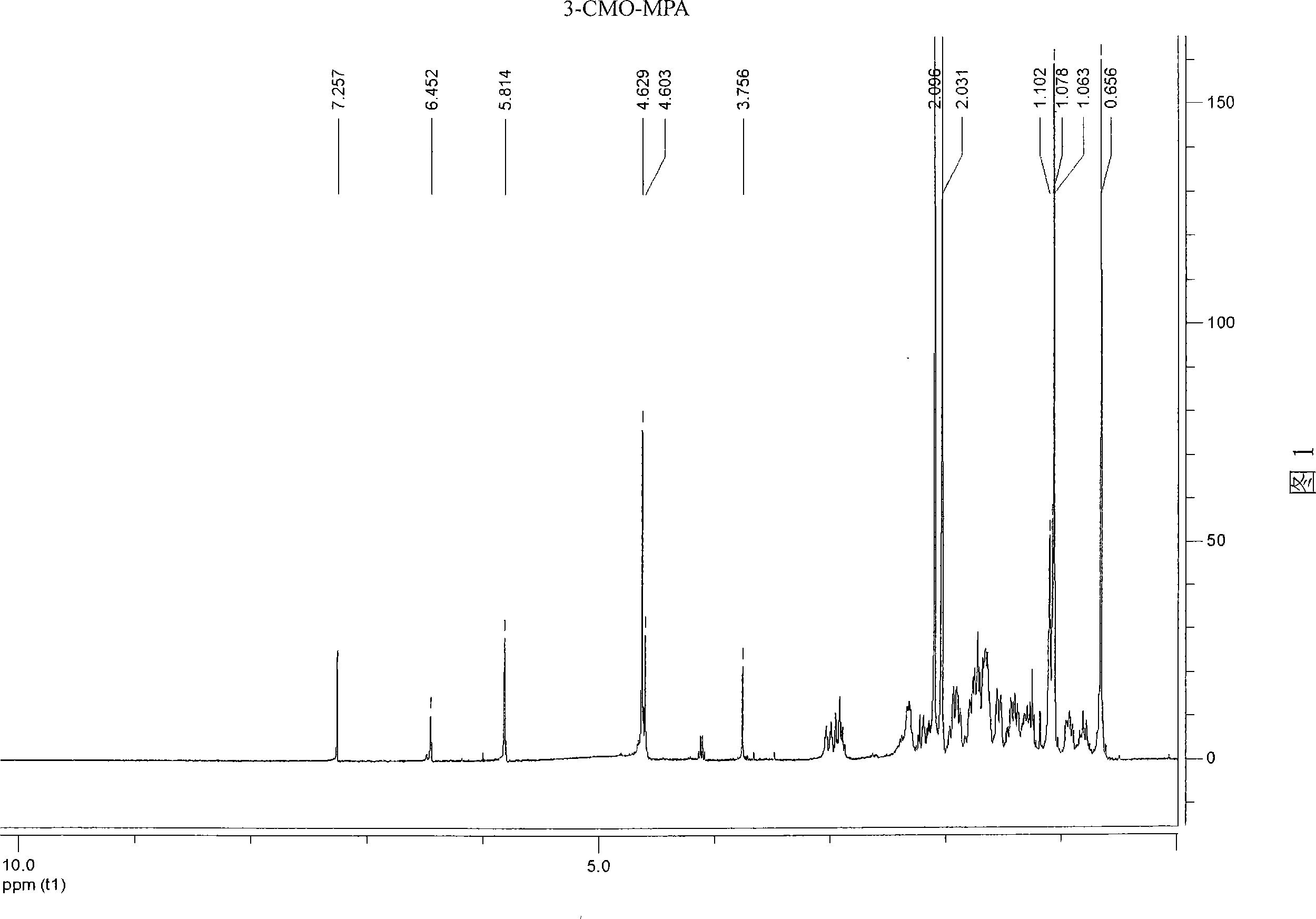
[0031] Example 1: Synthesis of artificial hapten 3-carboxymethyloximino-6-methyl-17α-hydroxyl-pregnant-20-one acetate (3-CMO-MPA)
[0032] Reaction formula:
[0033]
[0034] According to the feeding ratio medroxyprogesterone acetate (MPA): carboxymethyloxamine hydrochloride=1: 1.2mol ratio, take by weighing 1mmol MPA and dissolve in methanol, then 1.2mmol carboxymethyloxamine hydrochloride and An appropriate amount of sodium acetate was dissolved therein to make the pH 6, and stirred at room temperature for 24 hours. After methanol was distilled off under reduced pressure, the residue was extracted with ethyl acetate (3×20ml), and then 10g Na 2 SO 4 Dry, filter, distill to remove ethyl acetate, and dry to obtain a white solid which is 3-CMO-MPA.
[0035] The above products were taken by ESI and 1 H-NMR determined its structure. The ESI molecular ion peak of this material is 459 (M - -1), 1 H-NMR (CDCl 3 ) is: δ0.66(s, 3H, 18-CH 3 ); 1.06 and 1.10 (2s, 3H, E and Z ...
Embodiment 2
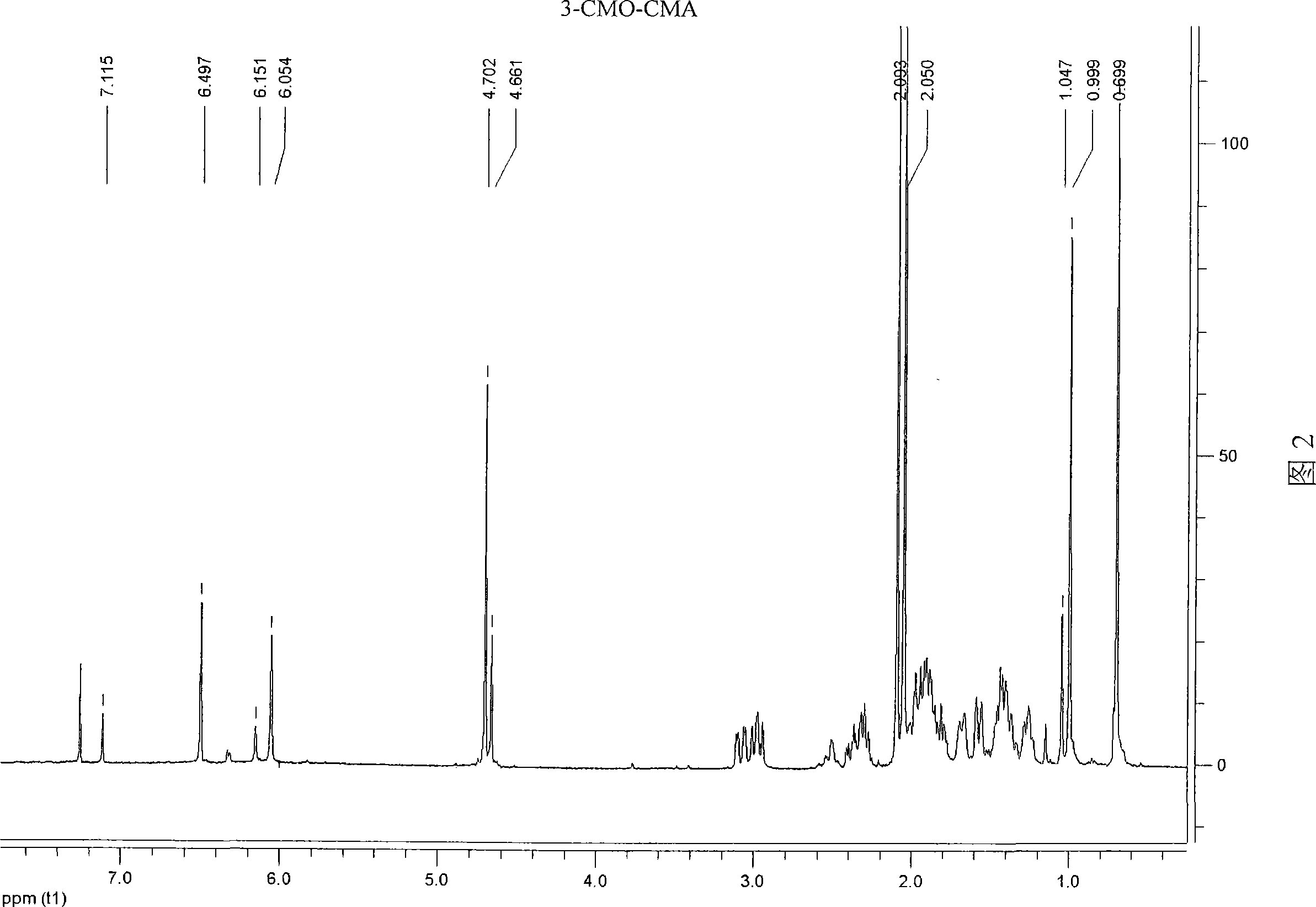
[0036] Example 2: Synthesis of artificial hapten 3-carboxymethyloximino-6-chloro-17α-hydroxyl-pregn-6-en-20-one acetate (3-CMO-CMA)
[0037] Reaction formula:
[0038]
[0039] According to the feeding ratio of chlormadinone acetate: carboxymethyloxamine hydrochloride=1: 1.5mol ratio, take by weighing 1mmolCMA and dissolve in methanol, then mix 1.5mmol carboxymethyloxamine hydrochloride and an appropriate amount of sodium acetate Dissolve in it, make pH 6, stir at room temperature for 24 hours. After methanol was distilled off under reduced pressure, the residue was extracted with ethyl acetate (3×20ml), and then weighed with 10g Na 2 SO 4Dry, filter, distill to remove ethyl acetate, and dry to obtain a white solid which is 3-CMO-CMA.
[0040] The structures of the above products were determined by ESI and 1H-NMR respectively. The ESI molecular ion peak of this material is 477 (M - -1), 1 H-NMR (CDCl 3 ) is: δ0.70(s, 3H, 18-CH 3 ); 1.00 and 1.05 (2s, 3H, E and Z 19-...
Embodiment 3
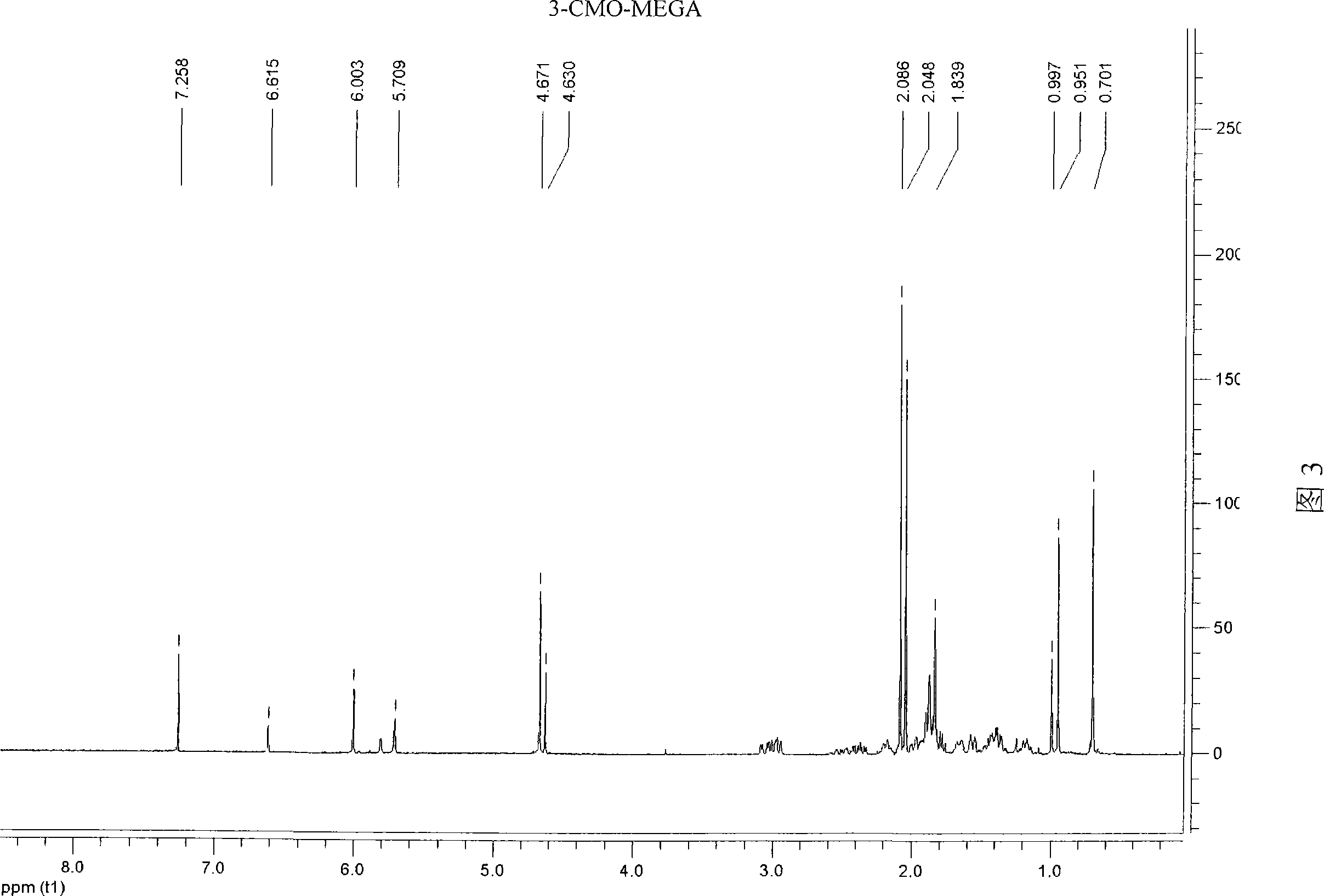
[0041] Example 3: Synthesis of artificial hapten 3-carboxymethyloximino-6-methyl-17α-hydroxyl-pregn-6-en-20-one acetate (3-CMO-MEGA)
[0042] Reaction formula:
[0043]
[0044] According to the feeding ratio of megestrol acetate: carboxymethyl oxamine hydrochloride = 1: 1.5mol ratio, take 1mmol MEGA and dissolve it in methanol, then mix 1.5mmol carboxymethyl oxamine hydrochloride and appropriate amount of sodium acetate Dissolve in it, make pH 6, stir at room temperature for 24 hours. After methanol was distilled off under reduced pressure, the residue was extracted with ethyl acetate (3×20ml), and then weighed with 10g Na 2 SO4 was dried, filtered, ethyl acetate was distilled off, and dried to give a white solid as 3-CMO-MEGA.
[0045] The structures of the above products were determined by ESI and 1H-NMR respectively. The ESI molecular ion peak of this material is 477 (M - -1), 1 H-NMR (CDCl 3 ) is: δ0.70(s, 3H, 18-CH 3 ); 0.95 and 1.00 (2s, 3H, E and Z 19-CH 3 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com