Process for the polymerisation of vinyl-containing monomers
A polymerization method, vinyl technology, applied in the direction of chemical recycling, etc., to achieve a well-controlled effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] S-PVC, K value 70
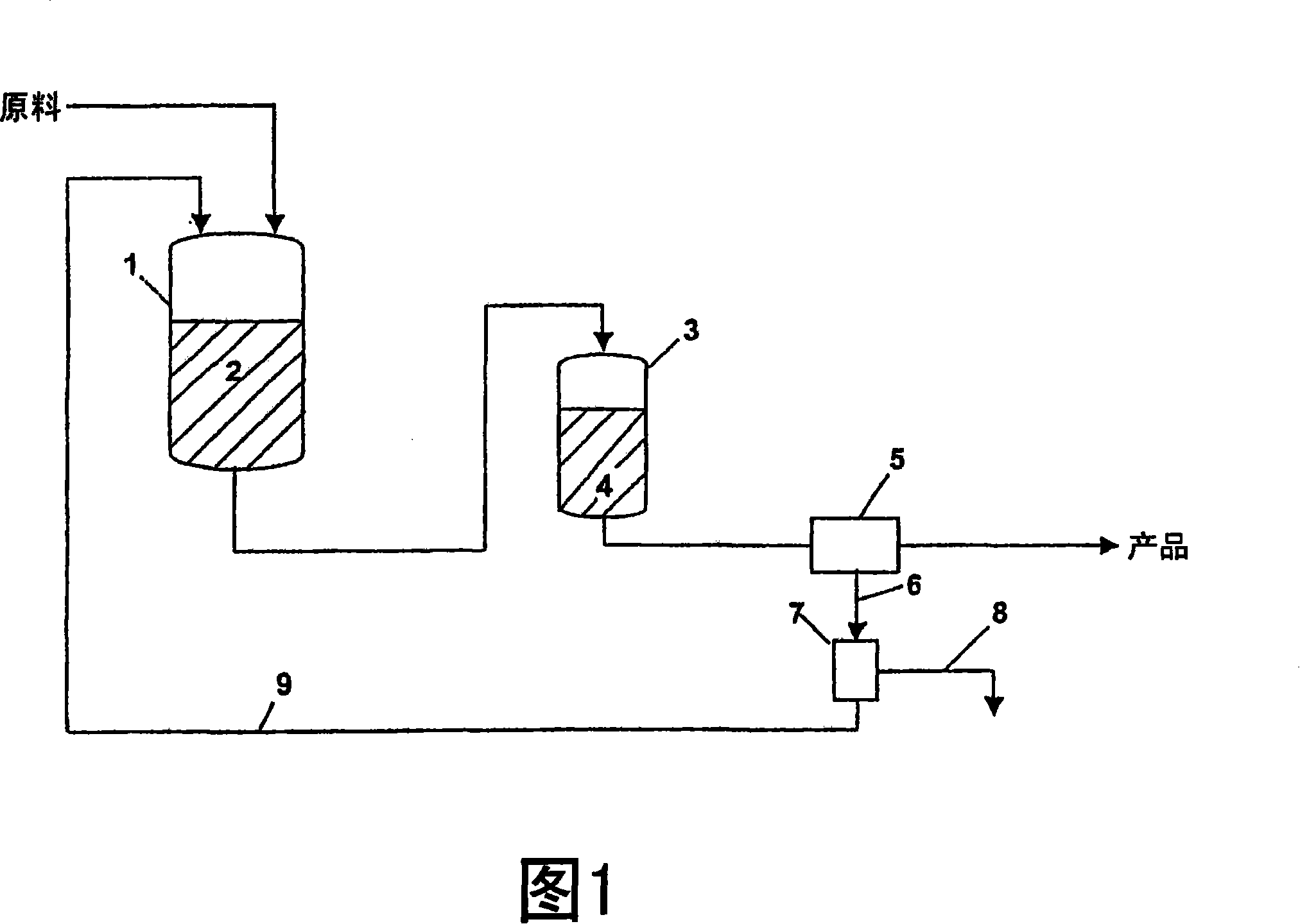
[0042] Polymerization of vinyl chloride was carried out in an aqueous reaction mixture at a temperature of 53° C. in a reactor using the method shown in FIG. 1 . After degassing, the resulting reaction mixture was mechanically dehydrated in a centrifuge and the polymer product was separated off. The reaction mixture from the centrifuge was filtered through a conventional plate module. The filtered solids were removed and the filtered reaction mixture was returned to the reactor and reused for further polymerization. Repeat the steps described several times for this PVC type. The quality of the filtered reaction mixture (Table 3) and the quality of the product (Table 4) obtained as a result of using the reaction mixture several times varied only very little; in particular the powder properties of the product did not produce any significant changes.
[0043] Table 3: Test data for filtered reaction mixture
[0044] PVC type: S-PVC, K valu...
Embodiment 2
[0048] S-PVC, K value 68
[0049] Polymerization of vinyl chloride was carried out in an aqueous reaction mixture at a temperature of 53° C. in a reactor using the method shown in FIG. 1 . After degassing, the resulting reaction mixture was mechanically dehydrated in a centrifuge and the polymer product was isolated. The reaction mixture from the centrifuge was filtered through a conventional plate module. The filtered solids were removed and the filtered reaction mixture was returned to the reactor and reused for further polymerization. Repeat the steps described several times for this PVC type. The quality of the filtered reaction mixture (Table 5) and the quality of the product (Table 6) resulting from using the reaction mixture several times varied only very slightly; in particular the powder properties of the product did not undergo any significant changes.
[0050] Table 5: Test data for filtered reaction mixture
[0051] PVC type: S-PVC, K value 68
un...
Embodiment 3
[0055] S-PVC, K value 70
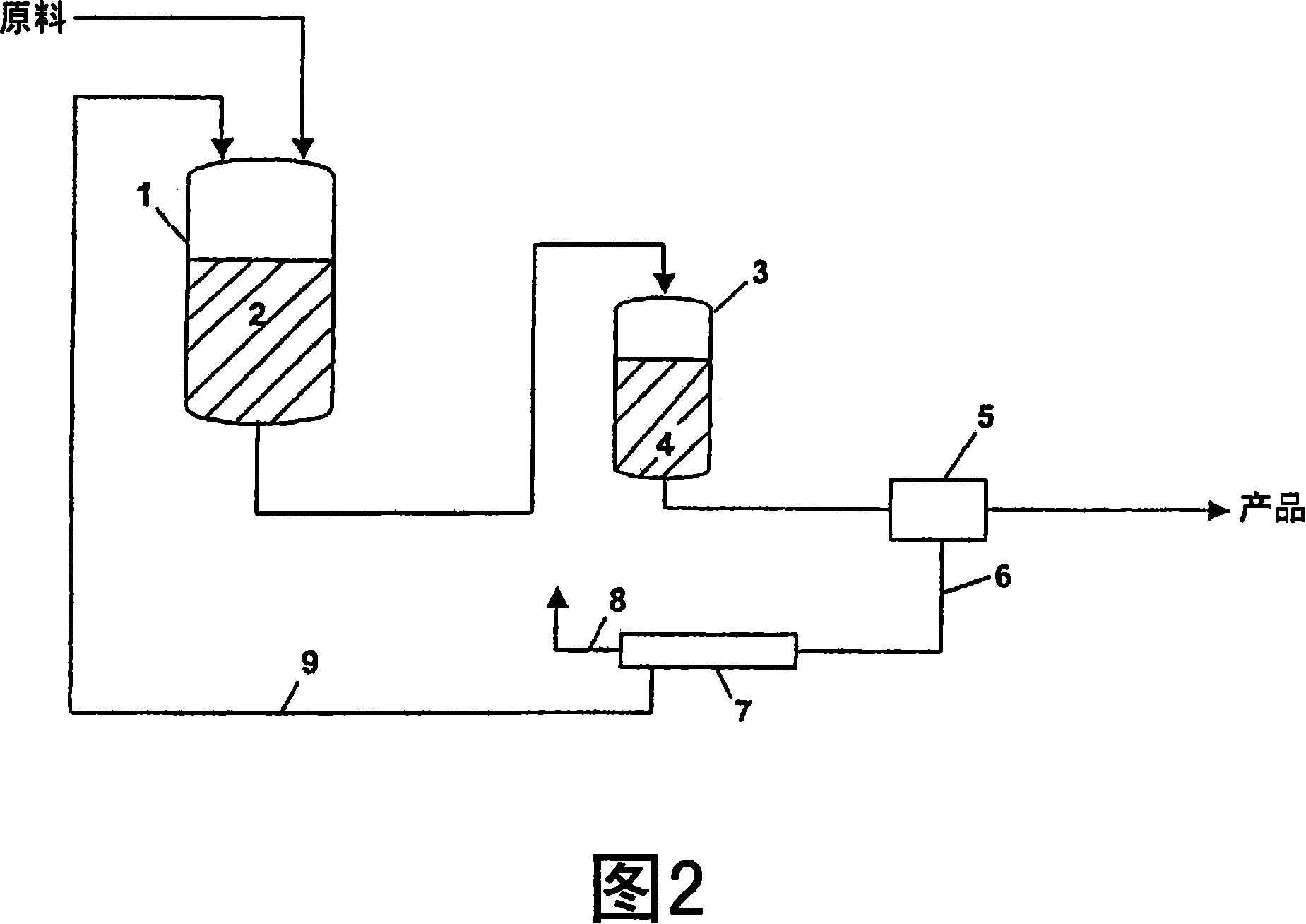
[0056] Polymerization of vinyl chloride was carried out in an aqueous reaction mixture at a temperature of 53° C. in a reactor using the method shown in FIG. 2 . After degassing, the resulting reaction mixture was mechanically dehydrated in a centrifuge and the polymer product was isolated. The reaction mixture from the centrifuge was filtered through a conventional tubular module. The filtered solids were removed and the filtered reaction mixture was returned to the reactor and reused for further polymerization. Repeat the steps described several times for this PVC type. The quality of the filtered reaction mixture (Table 7) and the quality of the product (Table 8) resulting from using the reaction mixture several times varied only very slightly; in particular the powder properties of the product did not undergo any significant changes.
[0057] Table 7: Test data for filtered reaction mixture
[0058] PVC type: S-PVC, K value 70
u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com