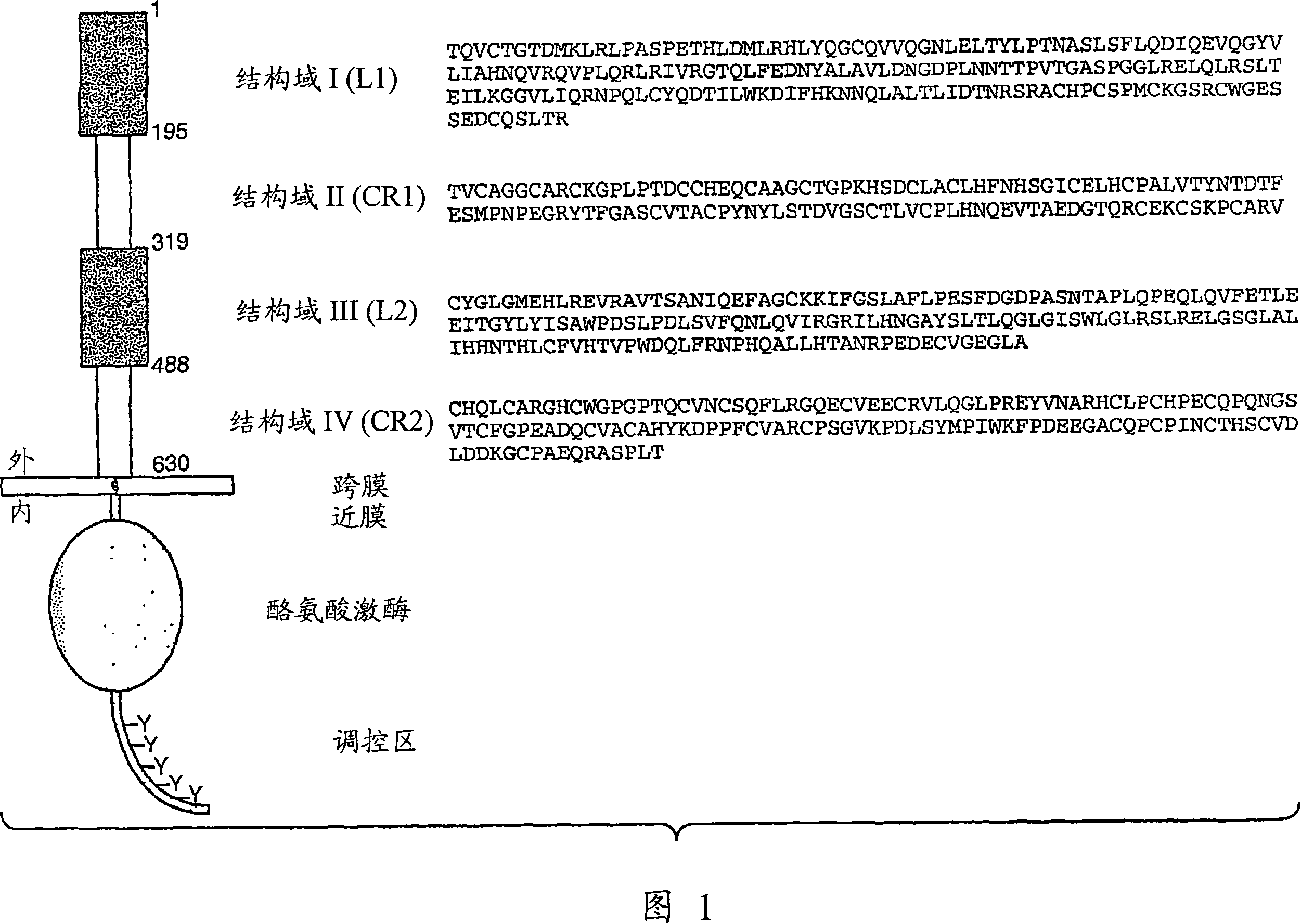
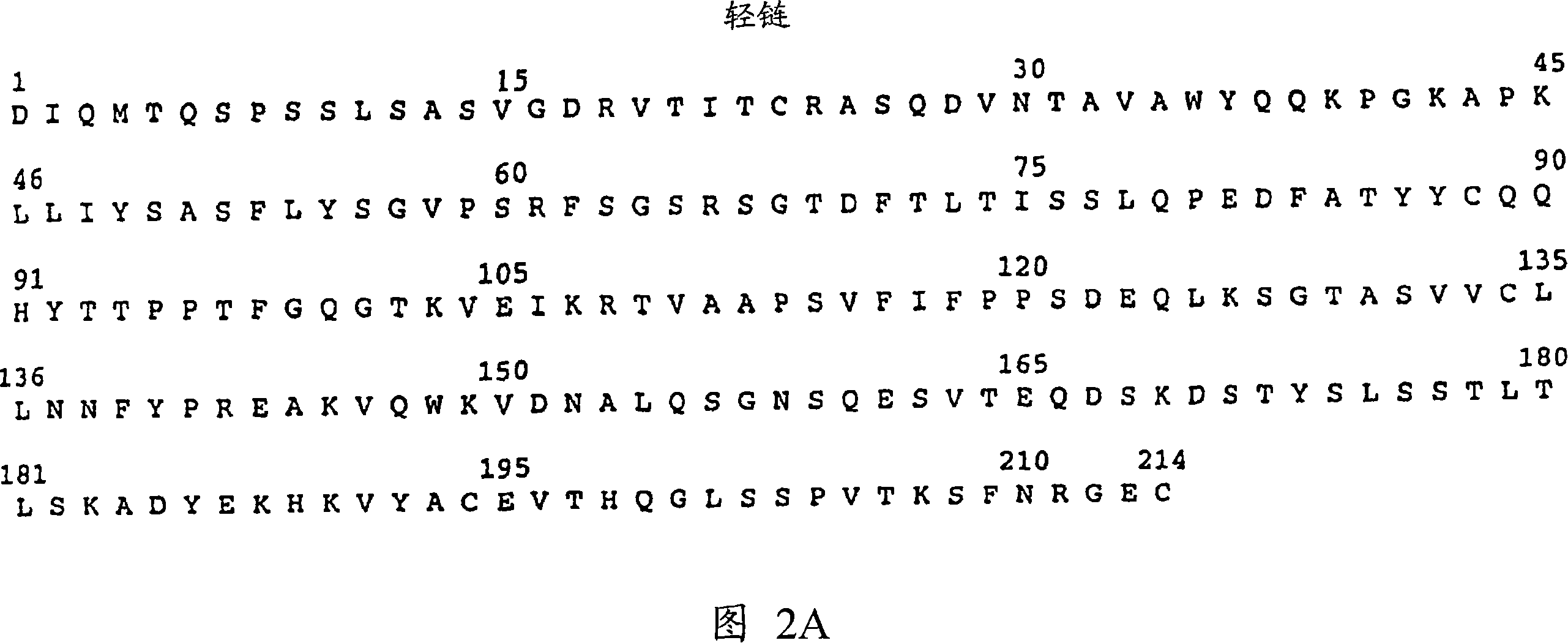
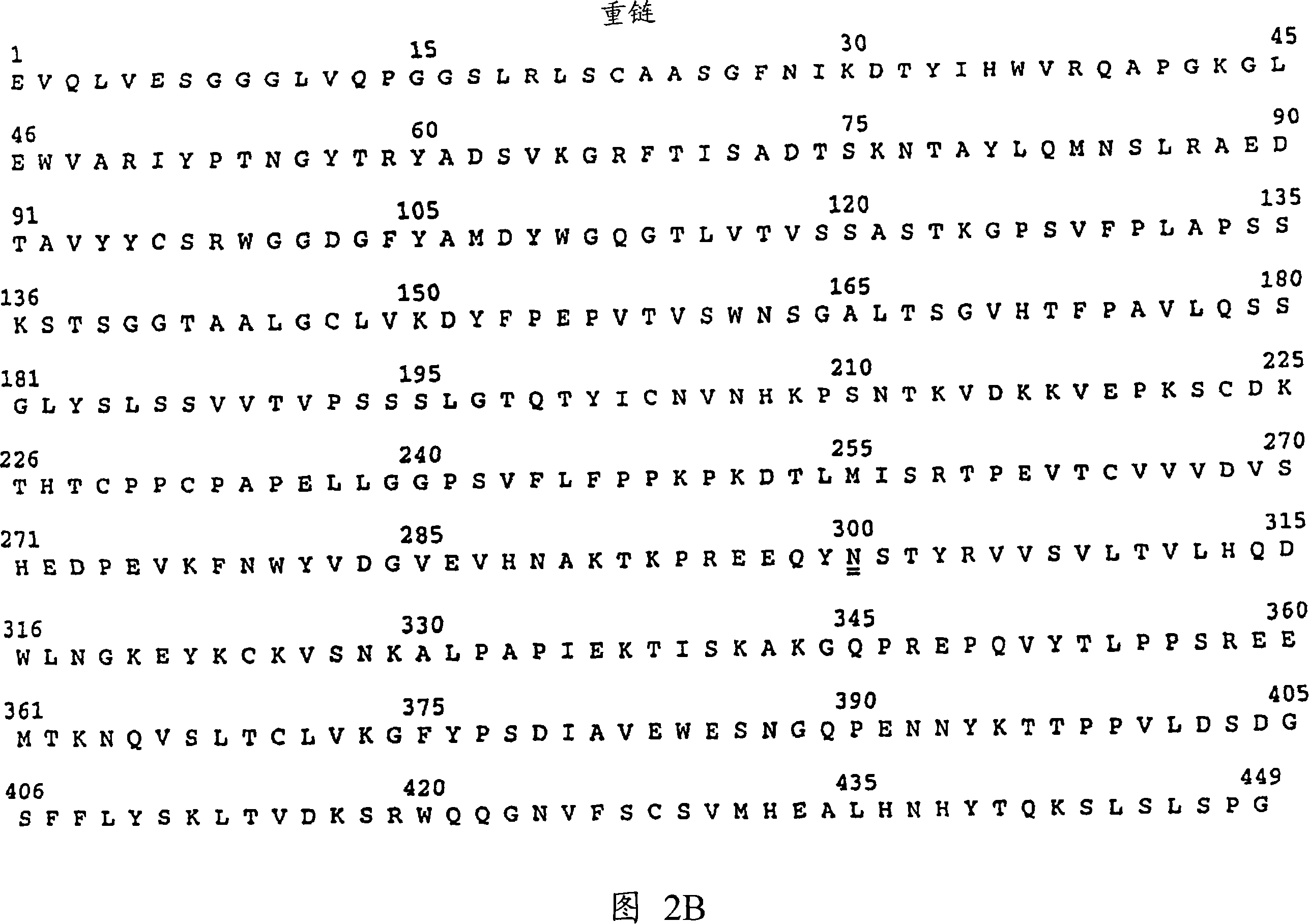
Inhibiting her2 shedding with matrix metalloprotease antagonists
A matrix metal, antagonist technology, applied in the direction of immunoglobulin, anti-animal/human immunoglobulin, antibody, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0253] The examples below investigate the role of matrix metalloproteinases (MMPs) in HER2 shedding.
[0254] Materials and methods
[0255] Cell Culture and Transfection
[0256] All cell lines used in this study were obtained from the American Type Culture Collection (ATCC, Manassas, VA). BT 474, MCF-7 and SKBR-3 cells were maintained in high glucose DMEM:Ham's F12 (50:50) supplemented with 10% heat-inactivated FBS and 2 mM L-glutamine. Cos-7 cells were maintained in high glucose DMEM supplemented with 10% heat-inactivated FBS and 2 mM L-glutamine. Cell lines were supplied with 5% CO 2 maintained at 37 °C in a humidified incubator. BT 474 and SKBR-3 cells using kit V according to the manufacturer's instructions (AMAXA TM ) the transient transfection described in the electroporation method. Cos-7 cells using LIPOFECTAMINE TM 2000 (Invitrogen) according to the manufacturer's recommendations for transfection as described.
[0257] Fo5 and f2:1282 mouse xenograft tumor ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com