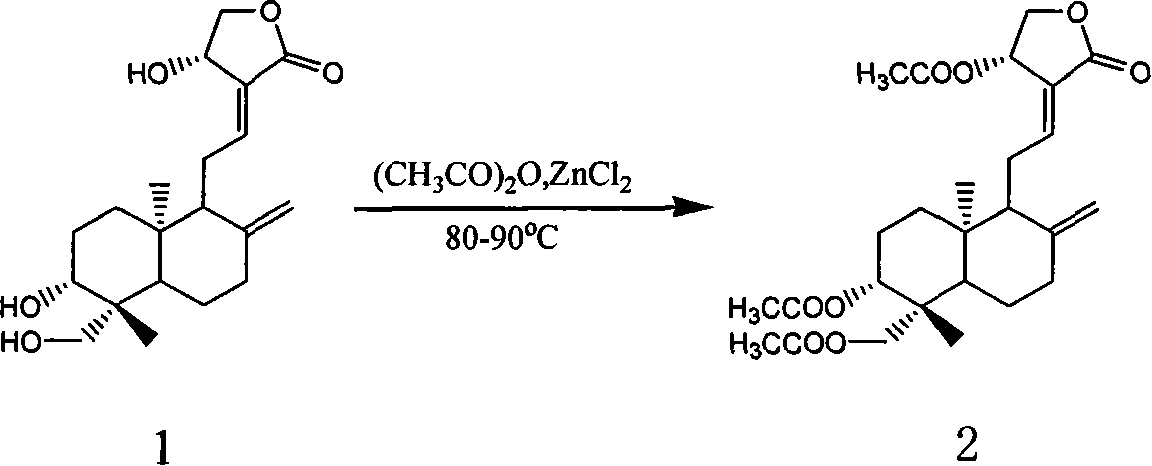Pharmaceutical composition containing triacetyl andrographolidume and medical use of the same
A technology of andrographolide and triacetyl, used in immunosuppressive and anti-tumor drugs, in the field of preparation of anti-inflammatory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0006] 1. Preparation of triacetylandrographolide
[0007]
[0008] Add 5g (14.3mmol) of andrographolide (1), 20mL of acetic anhydride, and 2g of newly molten zinc chloride into a 100mL single-necked flask in sequence, attach a reflux condenser, and heat in an oil bath at 80°C. After the reaction solution was clarified, the reaction was stopped, and the reaction solution was poured into a beaker filled with 80 mL of ice water, stirred vigorously, filtered with suction, and the filter cake was washed with water to pH7. The filter cake was crystallized with ethanol to obtain 4.7 g of white needle crystals with a yield of 67.8%. m.p.128°C. IR (cm -1 ): 2976, 2955, 2925, 2868, 1753, 1736, 1680, 1450, 1375, 1251, 1026, 908. MS (ESI) m / z: 476.3M + , 477.3(M+1) + , 499.3 (M+Na) + . 1 H-NMR (CDCl 3 , δ): 7.01 (1H, t, J=5.2, 12-H), 5.91 (1H, d, J=6.0, 14-H), 4.90, 4.53, (each 1H, s, 17-H), 4.60 (1H, dd, J=6.1, 11.2, 15-H), 4.55 (1H, dd, J=5.0, 11.2, 15-H), 4.38, 4.14 (each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com