Modified transferrin fusion proteins
A fusion protein, transferrin technology, applied in transferrin, animal/human proteins, hybrid peptides, etc., can solve problems such as unmodified or modified
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0543] By fusing one or more copies of the nucleotide sequence encoding the anti-fusion HIV-1 peptide (T-20) with the nucleotide sequence of TF, a fusion protein containing the modified Tf of the sequence and the peptide is prepared, The resulting fusion protein has a peptide fused to the N- or C-terminus of Tf.
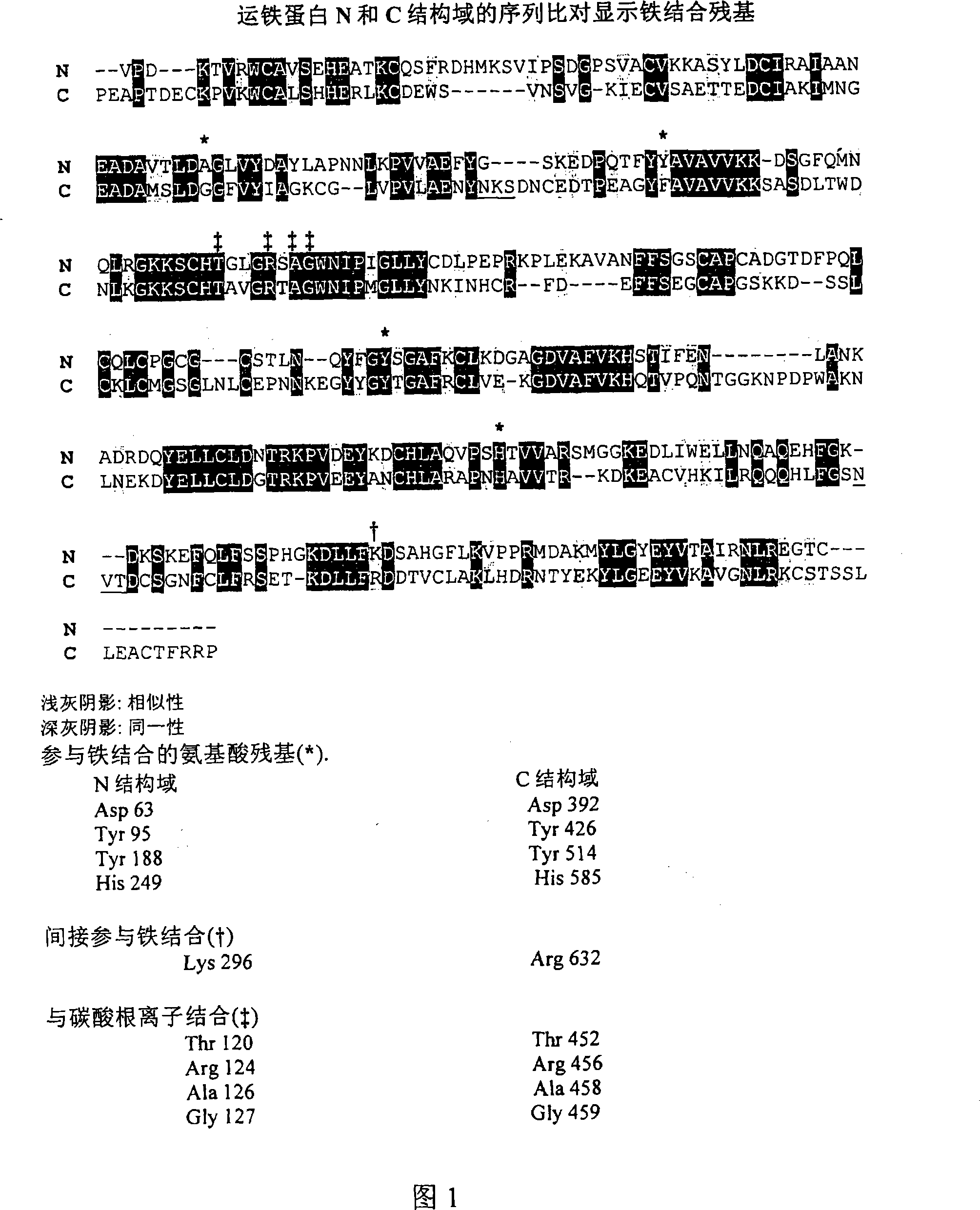
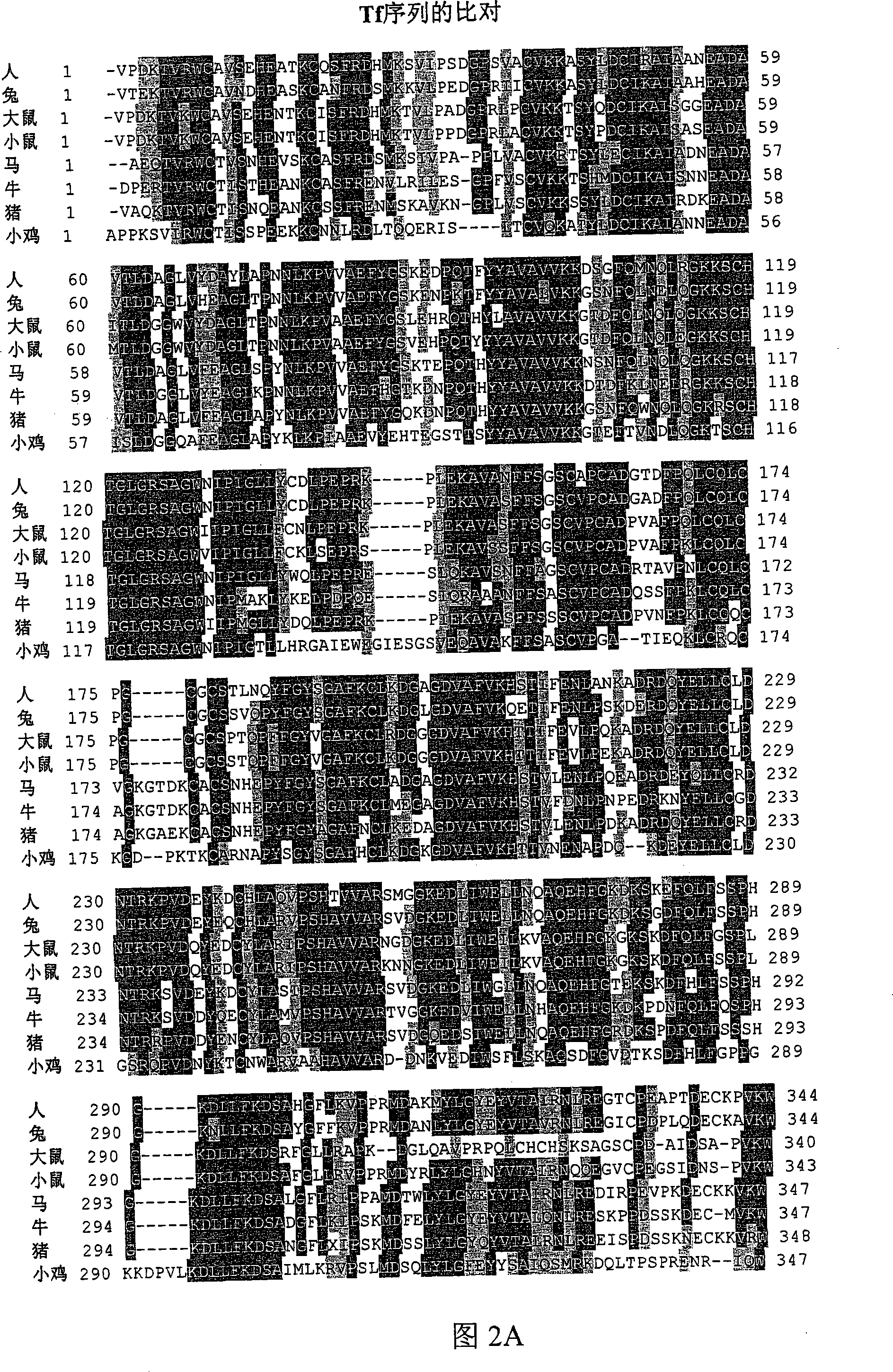
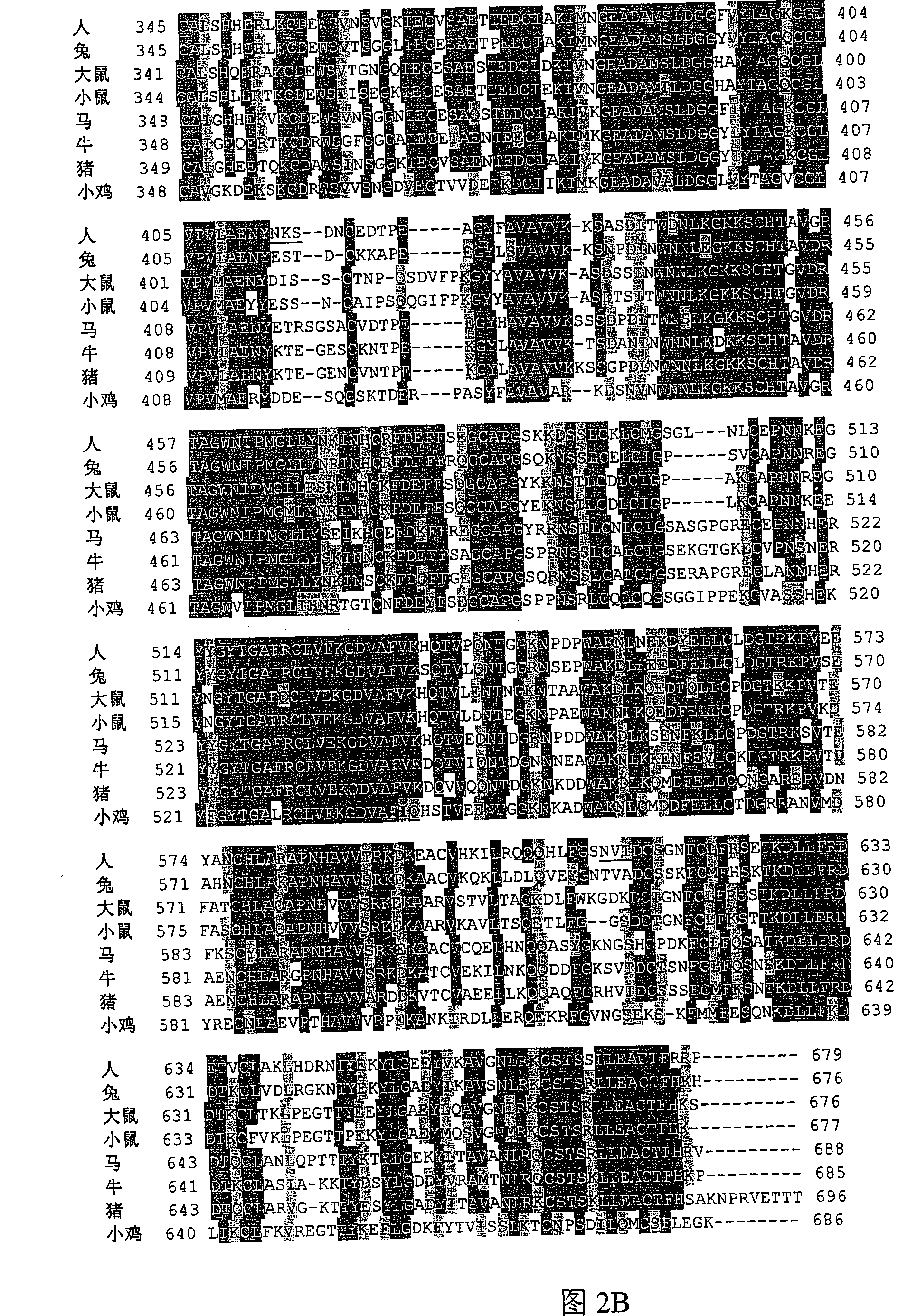
[0544] In one embodiment, the Tf portion of the fusion protein is engineered so that it is not glycosylated when produced in yeast. As mentioned above, human transferrin has two N-linked glycosylation sites at approximately N413 and approximately N611. N-linked glycosylation sites contain the sequence N-X-S / T. In one embodiment, N(Asn) is changed to Q(Gln); other changes can also be made, such as Asn to Ala or Ser to any other amino acid.
[0545] Specifically, N413 and N611 codons were converted to GAT and GAC by oligonucleotide-directed mutagenesis using the dut- and ung-method. See Kunkel et al. (1985) Proc. Natl. Acad. Sci. 82:488-492. Mutagenic oligonucleoti...
Embodiment 2
[0589] INGAP fusions were prepared using the reverse translated human INGAP amino acid sequence. The protein sequence is as follows: sp|Q92778|PBCG_HUMAN Human INGAP
[0590] MMLPMTLCRMSWMLLSCLMFLSWVEGEESQKKLPSSRITCPQGSVAYGS
[0591] YCYSLILIPQTWSNAELSCQMHFSGHLAFLLSTGEITFVSSLVKNSLTAYQY
[0592] IW[IGLHDPSHGTLPNG]GWKWSSSNVLTFYNWERNPSIAADRGYCAVLS
[0593] QKSGFQKWRDFNCENELPYICKFKV (SEQ ID NO: 17)
[0594] Back translation into DNA (codons optimal for yeast) gave the following sequences (SEQ ID NO: 18 and 19).
[0595] 1 atgatgttgc caatgacttt gtgtagaatg tcttggatgt tgttgtcttg tttgatgttt
[0596] m m l p m t l c r m s w m l l s c l m f
[0597] 61 ttgtcttggg ttgaaggtga agaatctcaa aaaaaa ttgc catcttctag aattacttgt
[0598] l s w v e q e e s q k k l p s s r i t c
[0599] 121 ccacaaggtt ctgttgctta tggttcttat tgttattctt tgattttgat tccacaaact
[0600] p q g s v a y g s y c y s l i l i p g t
[0601] 181 tggtctaatg ctgaattgtc ttgtcaaatg catttttctg gtcatttggc ...
Embodiment 3
[0680] The peptides given below have been shown to mimic EPO activity by causing dimerization of the EPO receptor. The cyclic peptide has no homology to EPO. To be active, the peptide has to act with another peptide (ie as a dimer) so that the two copies of the receptor come close enough to form an active complex. For many peptides, peptide dimers have only a short half-life, which could benefit from an extended half-life by fusion with transferrin. In this example, two peptides were added to the transferrin backbone.
[0681] 1 ggtggtactt actcttgtca ttttggtcca ttgacttggg tttgtaagcc acaaggtggt
[0682] g g t y s c h f g p l t w v c k p q g g
[0683] SEQ ID NO: 24 and 25.
[0684] Peptides could be successfully added between His289 and Gly290 of transferrin as described in detail by Ali et al. The inherent repeat of the transferrin molecule (two domains mirroring each other) means that the peptide can be inserted between Glu625 and Thr626 in the repeat region of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com