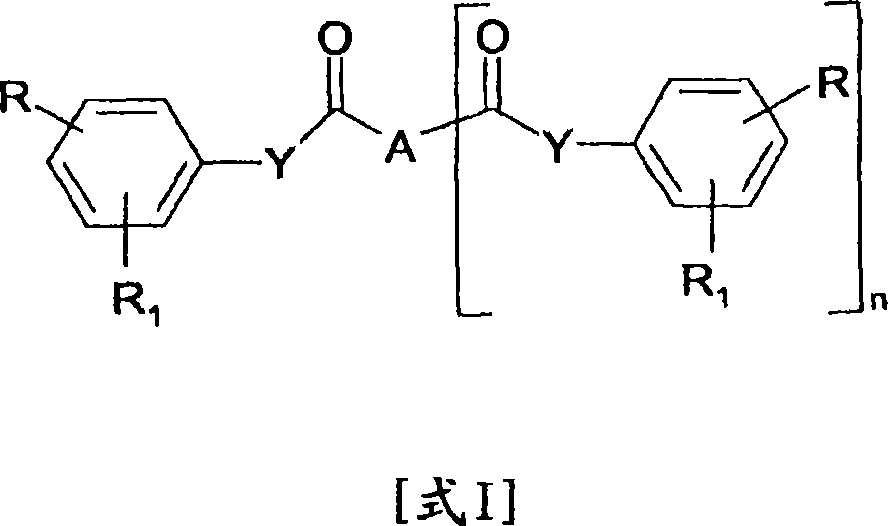
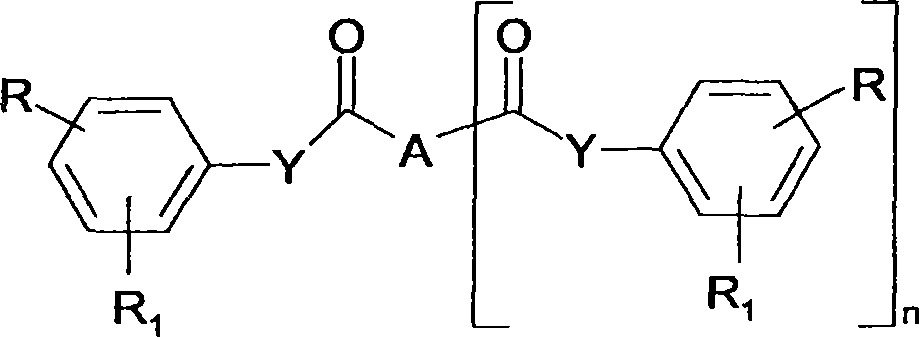
Cinnamic, phenylpropiolic and phenylpropanoic acid derivatives useful as anti-tumour agents
一种化合物、芳基的技术,应用在肉桂酸和苯丙炔酸和苯丙酸衍生物领域,能够解决抗肿瘤治疗无效等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] Preparation of (2E)-N-hydroxy-3-(4-{[(allyloxy)imino]methyl}phenyl)acrylamide (ST2984)
[0174] Step 1: Warm trans-4-formyl-cinnamic acid A (Y=CH=CH, R 1 = H, R 3 =H, 0.346 g, 1.96 mmol.) and B O-allylhydroxylamine hydrochloride (0.258 g, 2.37 mmol.) were dissolved in 2 mL of DMF and stirred for 5 h. Then, the solution was diluted with AcOEt and washed with water. in Na 2 SO 4 The organic layer was dried and then concentrated under reduced pressure to give 0.421 g of intermediate C(2E)-3-(4-{[(benzyloxy)imino]methyl}phenyl)acrylic acid (93% yield) .
[0175] MS (ESI) m / z: [M-1] - =230.3
[0176] Step 2: Intermediate C from Step 1 (0.123 g, 0.53 mmol) was dissolved in 1.5 mL DMF along with HATU (0.222 g, 0.58 mmol) and DIEA (185 μL, 1.06 mmol) in a flask. After 0.5 h, a solution of hydroxylamine hydrochloride (0.055 g, 0.80 mmol) and DIEA (139 μL, 0.80 mmol) in 1.5 mL DMF was added. The mixture was stirred at room temperature for 24 h, then diluted with HCl solu...
Embodiment 2
[0182] Preparation of (2E)-N-hydroxy-3-{4-[(phenoxyimino)methyl]phenyl}acrylamide (ST2985)
[0183] Step 1: Starting from trans 4-formyl-cinnamic acid A (0.342 g, 1.94 mmol) and B 0-phenylhydroxylamine hydrochloride (0.339 g, 2.33 mmol) as described in Example 1, Step 1, gave Intermediate C(2E)-3-{4-[(phenoxyimino)methyl]phenyl}acrylic acid used in the synthesis of ST2985 (0.520 g, 99% yield).
[0184] MS (ESI) m / z: [M-1] - =266.4
[0185] Step 2: Compound ST2985 (0.100 g, 48% yield) was obtained starting from Intermediate C (0.201 g, 0.75 mmol) as described in Example 1, Step 2.
[0186] MS(ESI)m / z: [M-1] - =281.1
[0187] [M+23] + =305.0
[0188] 1 H-NMR (200MHz, DMSO-d6) δ (ppm): 6.5-6.7 (d, J = 15.7Hz, 1H, CH), 7.0-7.1 (t, J = 6.9Hz, 1H, CH ar ), 7.2-7.3 (d, J=8.0Hz, 2H, 2×CH ar ), 7.3-7.4 (t, J=7.3Hz, 2H, 2×CH ar ), 7.4-7.6 (d, J=16.1Hz, 1H, CH), 7.6-7.7 (d, J=7.7Hz, 2H, 2×CH ar ), 7.8-7.9 (d, J=7.7Hz, 2H, 2×CH ar ), 8.7 (s, 1H, CH), 9.1 (...
Embodiment 3
[0191] Preparation of (2E)-N-hydroxy-3-[4-({[(4-nitrobenzyl)oxy]imino}-methyl)phenyl]acrylamide (ST2987)
[0192] Step 1: As described in step 1 of Example 1, from trans-4-formyl-cinnamic acid A (0.348g, 1.97mmol) and B 0-(4-nitrobenzyl) hydroxylamine hydrochloride (0.485g, 2.37 mmol) to give intermediate C(2E)-3-[4-({[(4-nitrobenzyl)oxy]imino}-methyl)phenyl]acrylic acid (ST3075) for the synthesis of ST2987 (0.610 g, 94% yield).
[0193] MS(ESI)m / z: [M-1] - =325.3
[0194] 1 H-NMR (200MHz, DMSO-d6) δ (ppm): 5.34 (s, 2H, CH 2 ), 6.5-6.7 (d, J=15.7Hz, 1H, CH), 7.5-7.8 (m, 7H, 6×CH ar , CH), 8.2-8.3 (d, J=8.4Hz, 2H, 2×CH ar ), 8.41(s, 1H, CH), 12.4(bs, 1H, OH).
[0195] 13 C-NMR (50MHz, DMSO-d6, δ): 75.0, 121.0, 124.3, 128.1, 129.4, 129.5, 133.8, 136.7, 143.7, 146.5, 147.7, 150.3, 168.2.
[0196] Step 2: Compound ST2987 (0.120 g, 44% yield) was obtained starting from Intermediate C (0.262 g, 0.80 mmol) as described in Example 1, Step 2.
[0197] MS(ESI)m / z: [M-1] - =34...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com