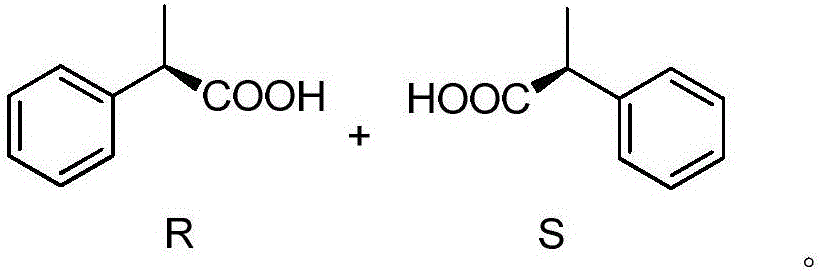A method for electrochemically synthesizing optically active 2-phenylpropionic acid
An optically active, phenylpropionic acid technology, applied in electrolytic organic production, electrolytic components, electrolytic processes, etc., can solve the problem of high preparation cost, achieve the effects of low cost, convenient operation, and reduce air pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] a. Preparation of electrolyte
[0019] Mix 0.5mmol 1-chloro-1-phenylethane 75ul with 1mmol tetraethylammonium iodide 0.2572g and 0.025mmol chiral Co II -(R,R)(salen) catalyst 0.0151g and 0.129mol N,N-dimethylformamide 10mL were mixed into an electrolyte solution, and then placed in a one-chamber electrolytic cell with stainless steel as the cathode and magnesium rod as the anode; The 1-chloro-1-phenylethane, tetraethylammonium iodide, chiral Co II -(R,R)(salen) catalyst and N,N-dimethylformamide are analytically pure, in which: 1-chloro-1-phenylethane is the substrate, tetraethylammonium iodide is the supporting electrolyte, and Sex Co II -(R,R)(salen) is the catalyst, N,N-dimethylformamide is grade molecular sieve dried solvent.
[0020] b. Electrocarboxylation reaction
[0021] Under normal pressure, feed carbon dioxide into the electrolytic cell to saturation, and then use 1.0mA / cm 2 The electric carboxylation reaction is carried out at a constant current dens...
Embodiment 2
[0026] a. Preparation of electrolyte
[0027] Mix 0.5mmol 1-chloro-1-phenylethane 75ul with 1mmol tetraethylammonium iodide 0.2572g and 0.025mmol chiral Co II -(R,R)(salen) catalyst 0.0151g and 0.129mol N,N-dimethylformamide 10mL were mixed into an electrolyte solution, and then placed in a one-chamber electrolytic cell with platinum as the cathode and magnesium rod as the anode; The 1-chloro-1-phenylethane, tetraethylammonium iodide, chiral Co II -(R,R)(salen) catalyst and N,N-dimethylformamide are analytically pure, in which: 1-chloro-1-phenylethane is the substrate, tetraethylammonium iodide is the supporting electrolyte, and Sex Co II -(R,R)(salen) is the catalyst, N,N-dimethylformamide is grade molecular sieve dried solvent.
[0028] b. Electrocarboxylation reaction
[0029] Under normal pressure, feed carbon dioxide into the electrolytic cell to saturation, and then use 1.0mA / cm 2 The electric carboxylation reaction is carried out at a constant current density, an...
Embodiment 3
[0033] a. Preparation of electrolyte
[0034] Mix 0.5mmol 1-chloro-1-phenylethane 75ul with 1mmol tetraethylammonium iodide 0.2572g and 0.025mmol chiral Co II -(R, R) (salen) catalyst 0.0151g and 0.129mol acetonitrile 10mL are mixed into electrolytic solution, then put into the one-chamber electrolytic cell with stainless steel as cathode and magnesium rod as anode; The 1-chloro-1-benzene Ethane, tetraethylammonium iodide, chiral Co II -(R,R)(salen) catalyst and acetonitrile are analytically pure, in which: 1-chloro-1-phenylethane is used as substrate, tetraethylammonium iodide is used as supporting electrolyte, chiral Co II -(R,R)(salen) is catalyst, acetonitrile is grade molecular sieve dried solvent.
[0035]b. Electrocarboxylation reaction
[0036] Under normal pressure, feed carbon dioxide into the electrolytic cell to saturation, and then use 1.0mA / cm 2 The electric carboxylation reaction is carried out at a constant current density, and the carbon dioxide is intro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com