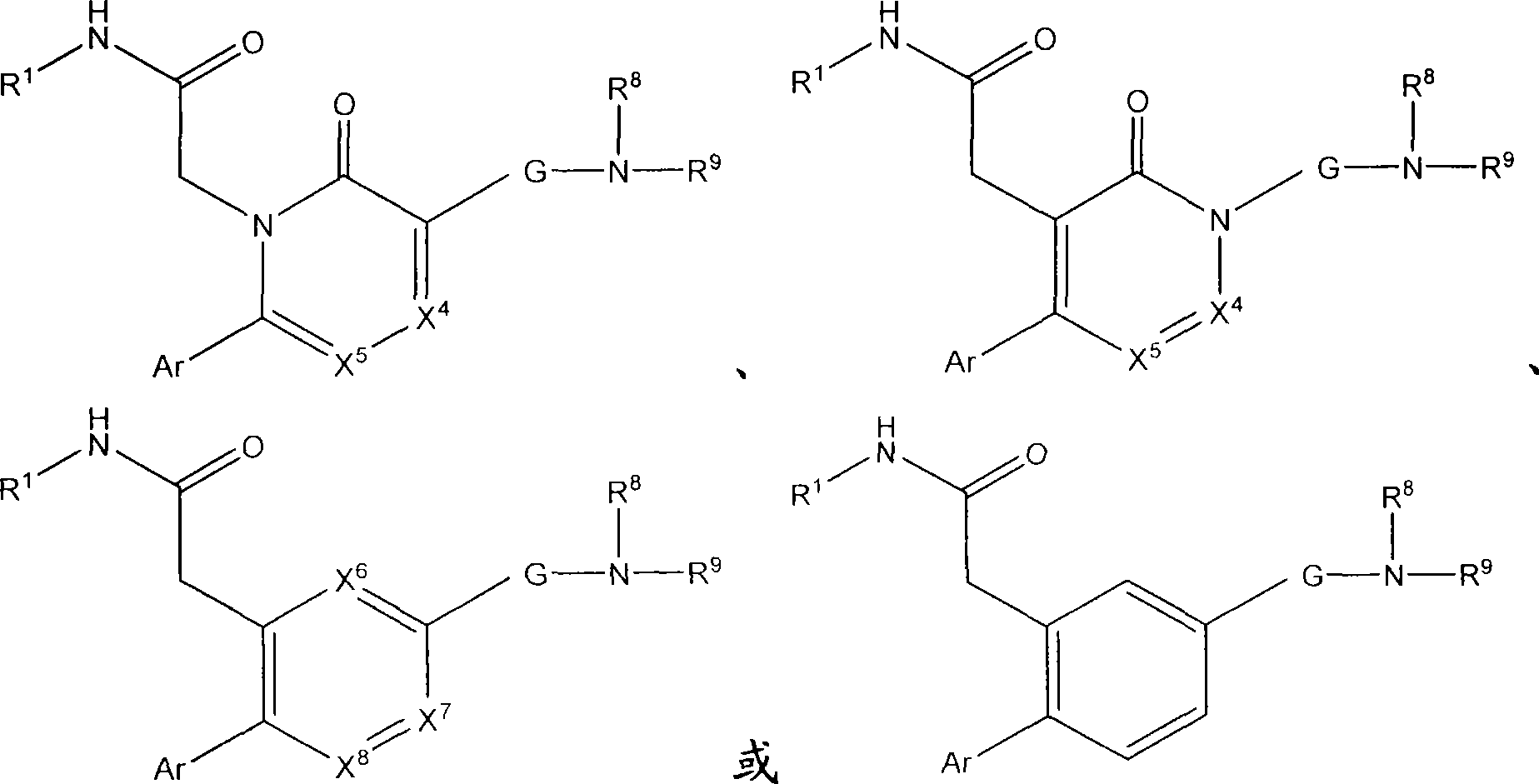
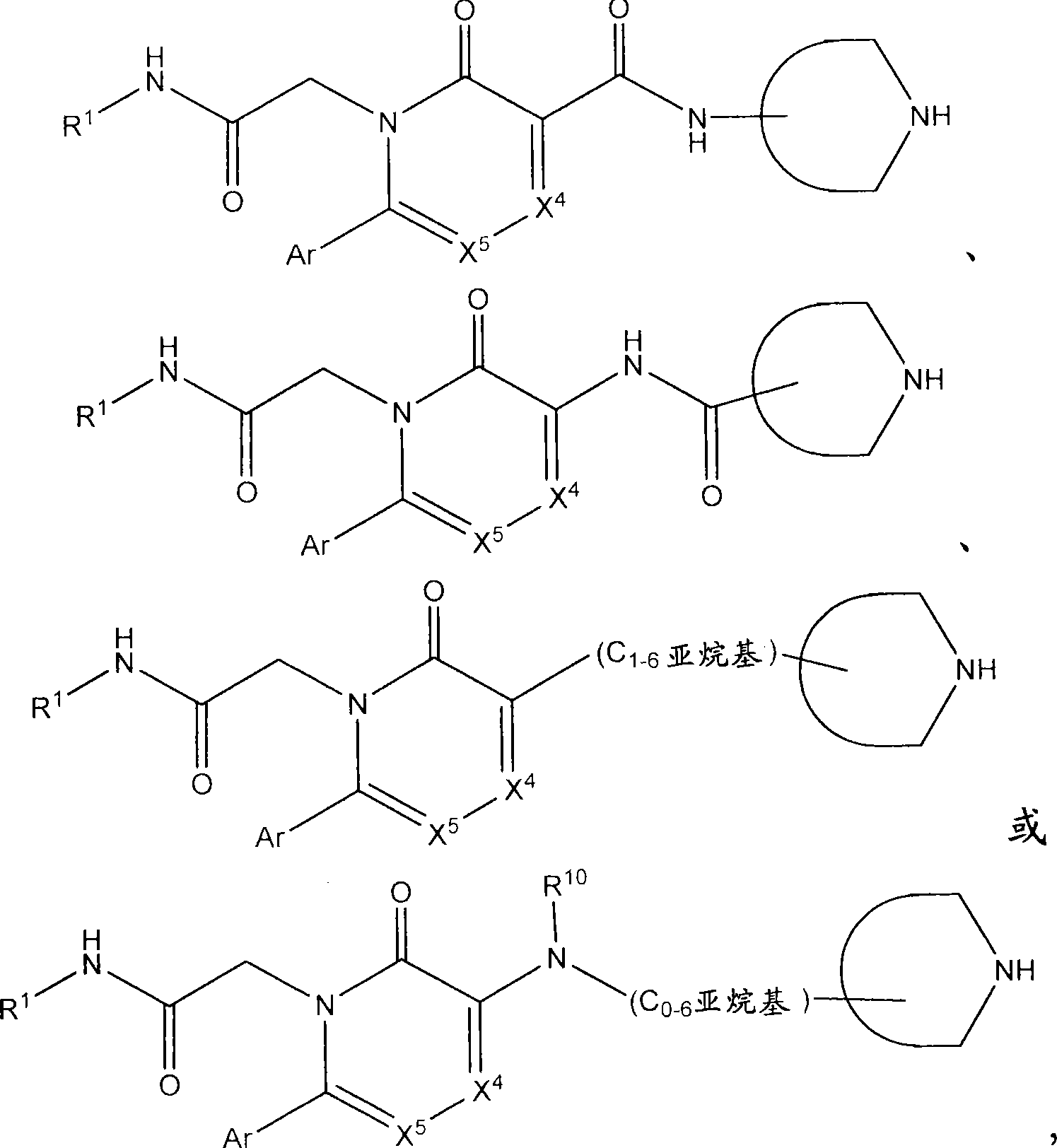
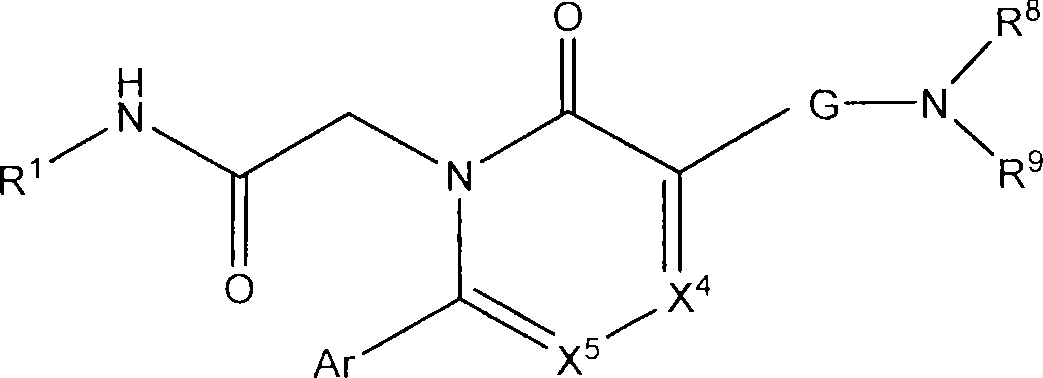
Azinone and diazinone V3 inhibitors for depression and stress disorders
A compound, selected technology, applied in medical preparations containing active ingredients, extracellular fluid diseases, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1. Synthesis of Intermediates 1-4
[0144]
[0145]
[0146] step 1 :
[0147] 3-Methoxyacetophenone (18.0g, 120.0mmol) and dimethylformamide dimethyl acetal (37.5g, 315mmol) were directly mixed in a microwave reactor and irradiated with microwave energy to 200°C for 10min. The reaction mixture was concentrated in vacuo and purified by flash column chromatography (SiO 2 ; eluting with 7:3 hexanes / EtOAc followed by 1:4.5:4.5 MeOH / EtOAc / hexanes), 26.0 g (100%) of enaminone 1-1 were collected as a dark orange-red oil.
[0148] 1-1 data: 1 H NMR (300MHz, CDCl3): δ7.75(d, 1H), 7.41(m, 2H), 7.23(d, 1H), 6.95(ddd, 1H), 5.64(d, 1H), 3.80(s, 3H ), 3.08 (br s, 3H), 2.87 (br s, 3H); MS (ESI), m / z (relative intensity, assigned) 206.1 (100, [M+H] + ).
[0149] step 2 :
[0150] To a solution of 1-1 (26.8 g, 130.5 mmol) in dimethylformamide (250 mL) were added sodium hydride (60%, 6.27 g, 261 mmol) and cyanoacetamide (11.0 g, 130.5 mmol). The mixture was the...
Embodiment 2
[0158] Example 2. Preparation 2-4
[0159]
[0160] step 1 :
[0161] To a solution of 1-4 (1 g; 3.75 mmol) in 80% EtOH(aq) (10 mL) was added KOH (843 mg; 15.02 mmol). The reaction mixture was then heated to reflux for 16h. The mixture was cooled to room temperature under H 2 Partition between O (100 mL) and EtOAc (50 mL). The aqueous phase was acidified to pH 3 using 2N HCl (aq) and extracted with EtOAc (3 x 50 mL). The combined organic phases were dried (MgSO 4 ), filtered and concentrated in vacuo to afford 990 mg (93%) of 2-1.
[0162] 2-1 data: 1 H NMR (300MHz, CDCl3): δ14.3(br s, 1H), 8.55(d, 1H), 7.41(dd, 1H), 7.08(dd, 1H), 6.92(d, 1H), 6.87(d, 1H), 6.50(d, 1H), 5.89(ddt, 1H), 5.23(d, 1H), 4.92(d, 1H), 4.62(d, 2H), 3.82(s, 3H); MS(ESI), m / z (relative intensity, assigned) 286.1 (79, [M+H] + ).
[0163] step 2 :
[0164] To a solution of 2-1 (223 mg; 0.78 mmol) in THF (5 mL) was added CDI (253 mg; 1.56 mmol). The reaction mixture was heated to 50 °C whi...
Embodiment 3
[0174] Example 3. Preparation 3-3
[0175]
[0176] step 1 :
[0177] To a solution of acid 2-1 (442 mg; 1.55 mmol) in THF (10 mL) was added CDI (0.51 g; 3.10 mmol). The reaction mixture was heated to 50 °C and stirred for 1.5 h. After the mixture was cooled, it was concentrated in vacuo and the crude residue was dissolved in EtOAc (100 mL) and H 2 O (50mL). The organic phase was dried (Na 2 SO 4 ), filtered and concentrated in vacuo.
[0178] NaH (60%; 65 mg; 1.63 mmol) was added to N'-hydroxy-3-(piperidin-1-yl)propionamidine (319 mg; 1.86 mmol) and 4 Å molecular sieves (1 scoop) in DMF (10 mL) The mixture was stirred at 23 °C for 30 min. To this was added the above imidazolide as a solution in DMF (3 mL w / 2 mL flush) via cannula. The reaction mixture was heated to 80 °C for 3 h. After the mixture was cooled, the H 2 Partition between O (100 mL) and 3:1 DCM / i-PrOH (3 x 40 mL). The combined organic phases were washed with brine (1×50 mL), dried (K 2 CO 3 ), f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com