Eslicarbazepine acetate and methods of use
A technology of eslicarbazepine acetate and drugs, which is applied in the direction of pharmaceutical formulas, medical preparations of non-active ingredients, digestive system, etc., and can solve problems such as side effects and reduced efficacy of oxcarbazepine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] The foregoing and following aspects and embodiments, including the studies discussed herein, have been described and illustrated by way of illustration only and should not be construed as limitations on scope.
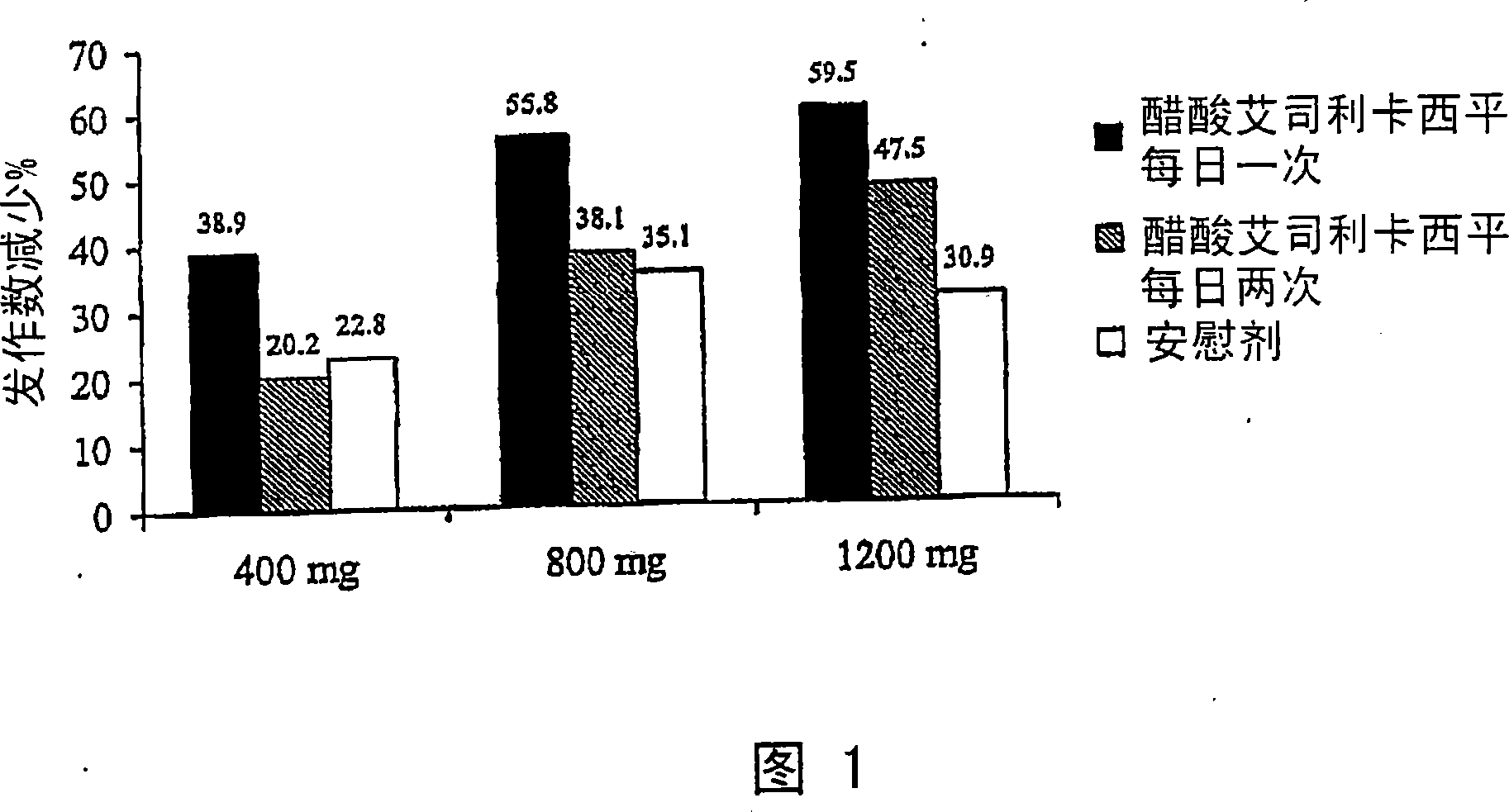
[0016] One aspect of the present disclosure pertains to a method of treating at least one disease or condition in a patient in need thereof by administering a pharmaceutical composition comprising a pharmacologically effective amount of eslicarbazepine acetate.
[0017] In an exemplary embodiment of the present disclosure, the pharmaceutical composition comprising eslicarbazepine acetate is administered in a once-daily dosing regimen.
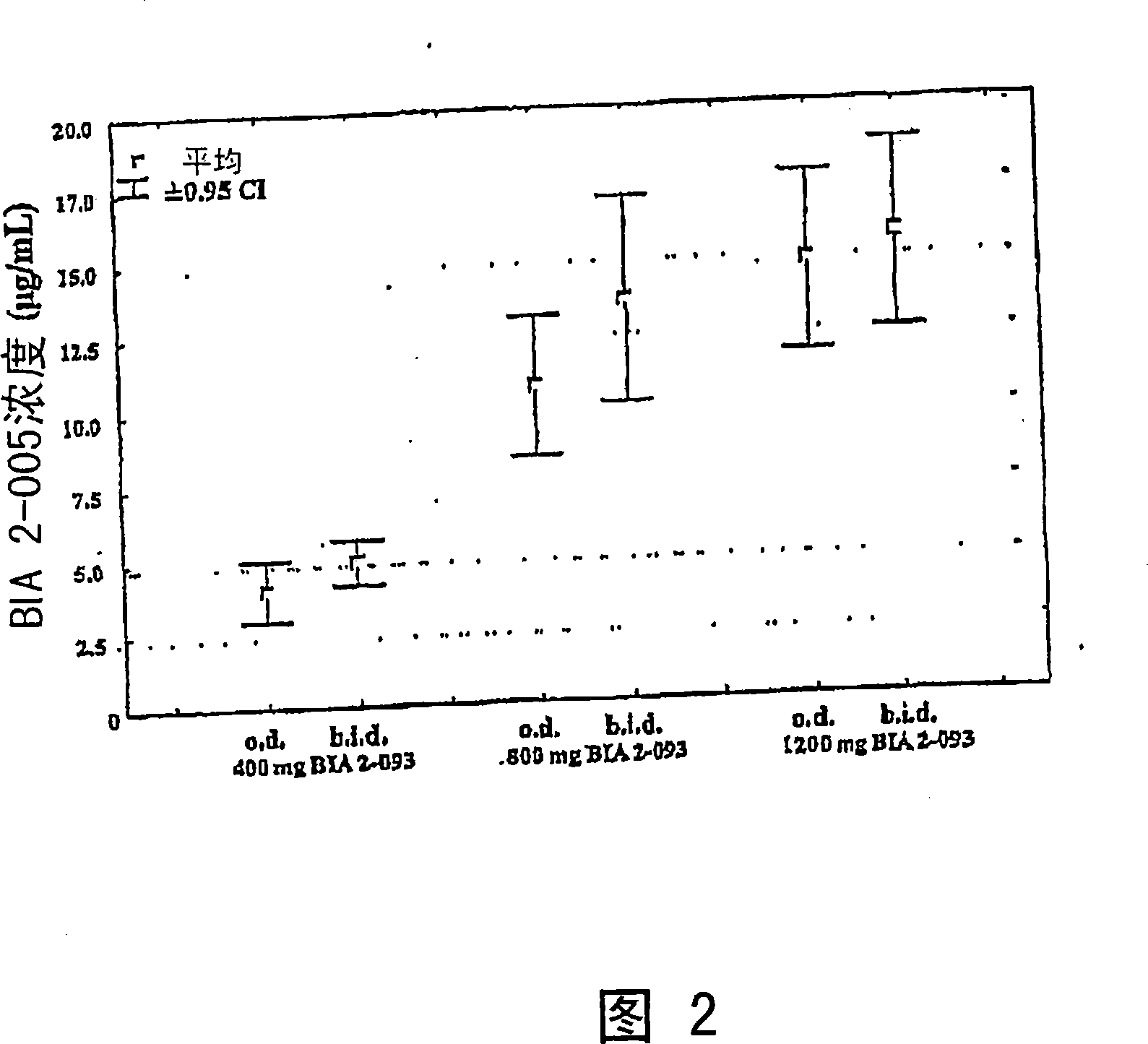
[0018] In another exemplary embodiment of the present disclosure, by exposure rate and exposure degree (C max and AUC 0-τ ) as a standard, the pharmaceutical composition is administered at a dose expected to maximize the total exposure to eslicarbazepine.
[0019] In an exemplary embodiment of the present disclosure, the at lea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com