2-phenylpyridine derivant with triphenylamine and carbazole as modification group and synthesizing method thereof
A technology of phenylpyridine and modified group, applied in the field of 2-phenylpyridine derivatives and synthesis thereof, to achieve the effects of simplifying multi-layer structure, improving transmission performance and reducing phosphorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
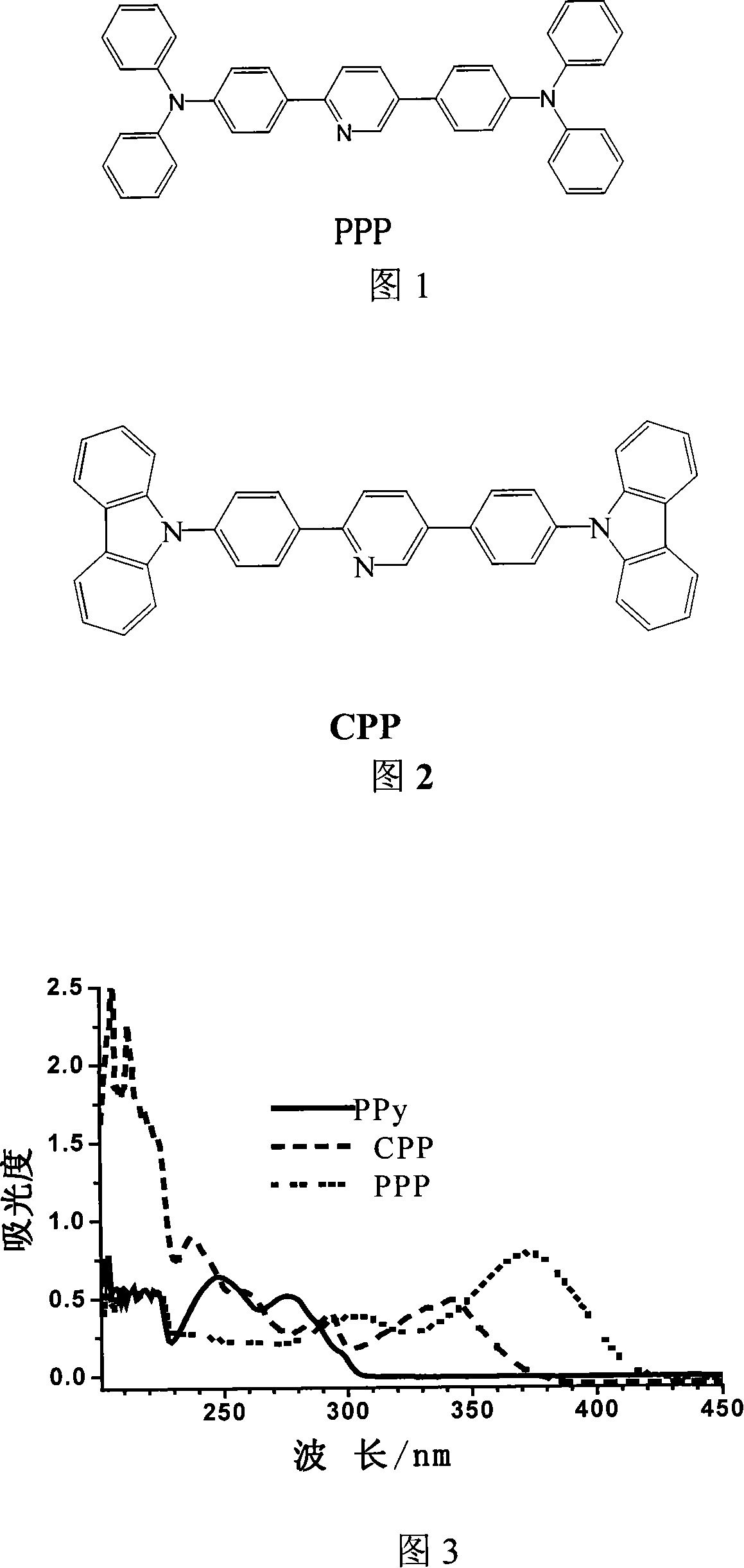
[0021] Embodiment 1: A 2-phenylpyridine derivative 2,5-bis((4'-N,N-diphenyl)phenyl)pyridine with triphenylamine as a modification group, the synthesis steps are as follows:
[0022] Synthesis of 2-aminopyridine (I):
[0023] Add 100mL of toluene and 30g (0.384mol) of crushed sodium amide into a 250mL three-necked flask, heat to 115°C, slowly add dry 40g (0.506mol) of pyridine dropwise, and reflux for 8h under the protection of nitrogen, Pour out a large amount of toluene, raise the temperature to 70°C, slowly add water dropwise, stir vigorously, separate the liquids, and spin dry to obtain a large amount of solids. After atmospheric distillation, the distillate is a white solid. After toluene recrystallization, a white flaky solid 33.6 g, the yield is 70%.
[0024] Synthesis of 2-amino-5-bromopyridine (II):
[0025] Add 8.46g (0.09mol) of 2-aminopyridine and 15mL of glacial acetic acid into a 150mL three-neck flask, cool to about 10°C, slowly add 4.62mL (0.09mol) of bromine ...
Embodiment 2
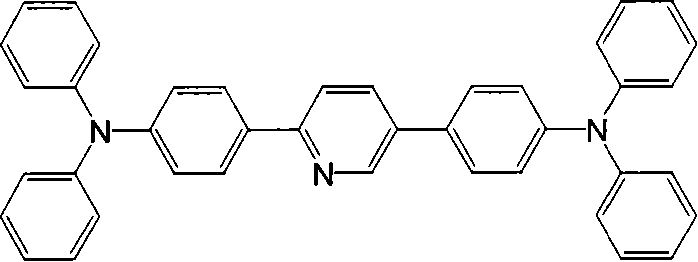
[0036] Embodiment 2: A kind of 2-phenylpyridine derivative 2,5-bis(4'-(9H-9-carbazolyl)phenyl)pyridine with triphenylamine and carbazole as the modification group, its synthesis steps as follows:
[0037] Synthesis of 2-aminopyridine (I):
[0038] Add 100mL of toluene and 30g (0.384mol) of crushed sodium amide into a 250mL three-necked flask, and under the protection of nitrogen, heat to 115°C, within 1h, slowly add 40.7mL (0.506mol) of dry pyridine dropwise, After reflux for 8h, the reaction was stopped. After cooling to room temperature, pour out a large amount of toluene, heat the obtained solid to 70°C, slowly add water dropwise, and stir vigorously. After all the solid is dissolved, separate the liquid while it is hot. It was purified by atmospheric distillation at 210°C, and the resulting fraction was cooled to a white solid, which was recrystallized with toluene to obtain 33.6 g of white flaky crystals, with a yield of 70%. m.p.56-58°C.
[0039] Synthesis of 2-amino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com