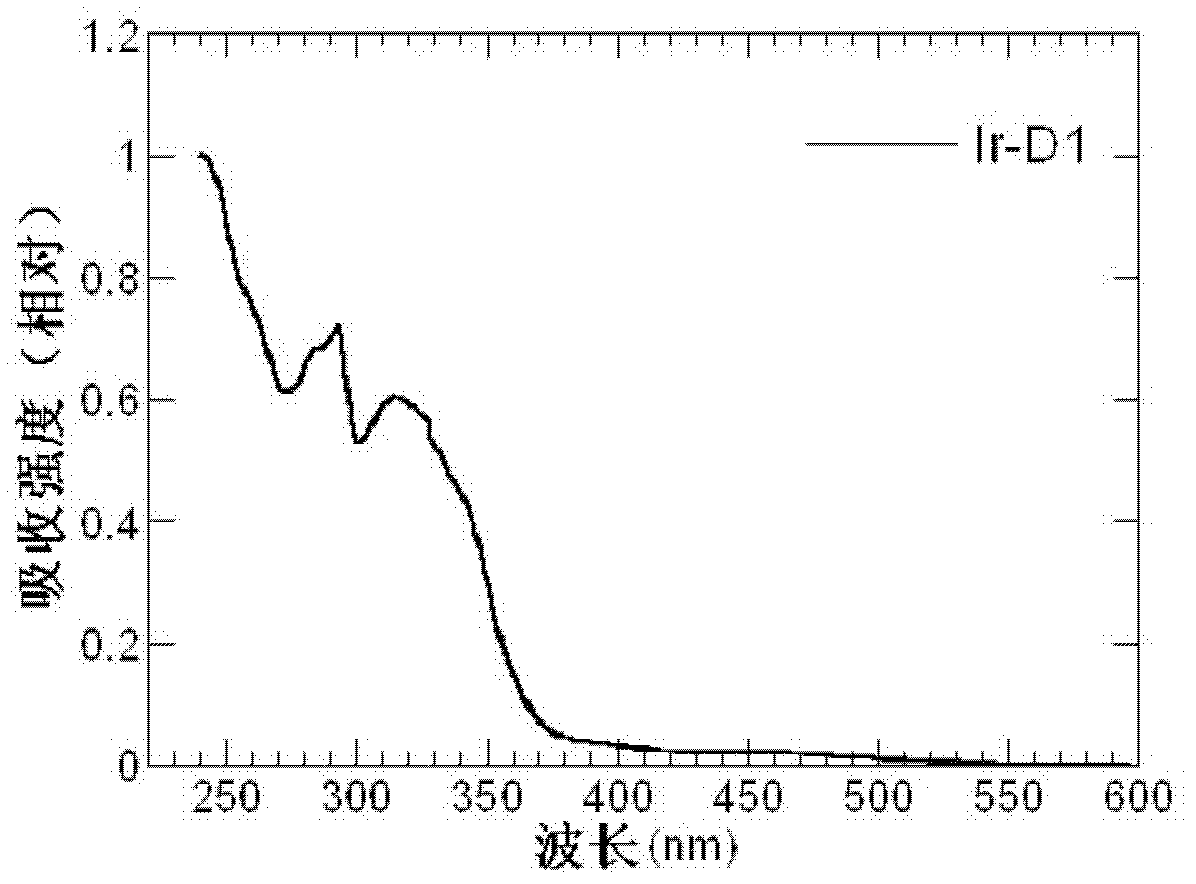
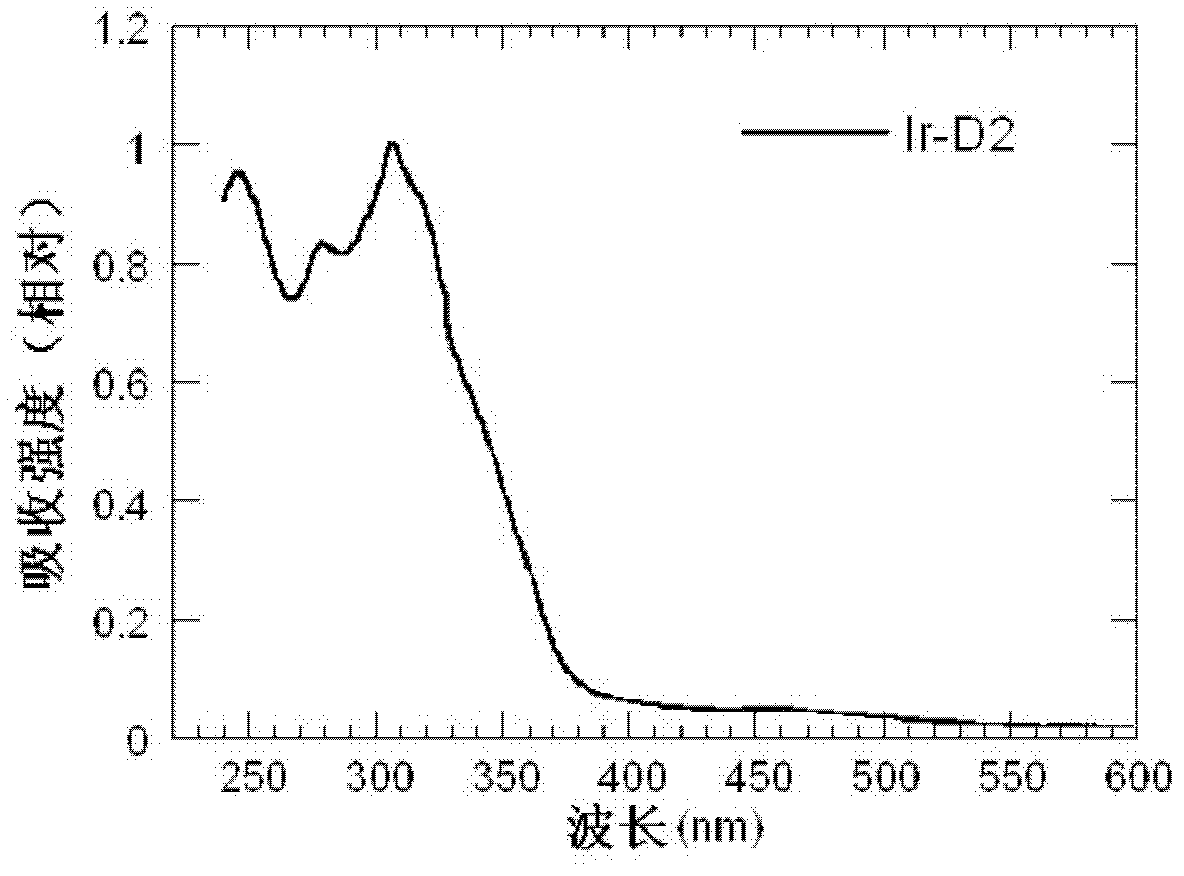
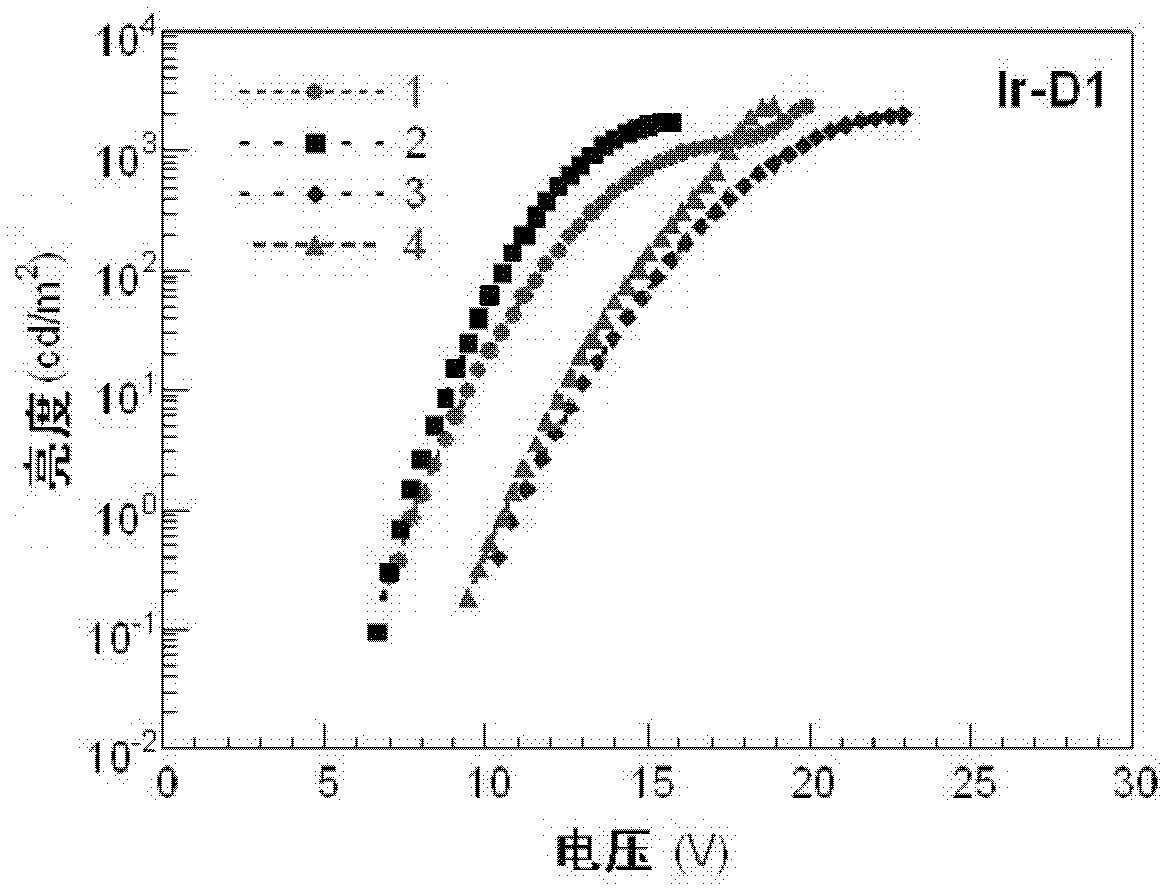
Dendritic iridium coordination compound with double-carrier transport property, application and prepared organic electrophosphorescent device
A technology of iridium complexes and transport properties, applied in the field of organic electroluminescence, can solve problems such as the difficulty in obtaining red electrophosphorescent materials with color purity, increased synthesis difficulty, and poor color purity, achieving good development prospects and short reaction times Short, Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] Synthesis of 1-(3-(9-carbazole)biphenyl)-3,4-dihydroisoquinoline (1) 6.50g (22.7mmol) 1-(3'-bromophenyl)-3,4 - dihydroisoquinoline and 9.22 g (25.0 mmol) N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborinyl)phenyl)carbazole, 12.6mL of 2M sodium carbonate solution, 70ml of toluene, and 35ml of ethanol were put into a 200ml three-necked flask, and Pd(PPh 3 ) 4 0.578g, reflux reaction for 24 hours, extracted with dichloromethane, washed with water several times, dried over anhydrous magnesium sulfate, rotovaped the solvent, passed the column with ethyl acetate:dichloromethane (1:9), and obtained a light yellow solid 4.46 g, yield 83.2%. GC-MS: m / z, 447.2, [M-1] + .1H NMR (300MHz, CDCl 3 ), (ppm): 8.17-8.14 (d, 2H), 7.96 (s, 1H), 7.88-7.85 (m, 2H), 7.81-7.78 (m, 1H), 7.66-7.55 (m, 4H), 7.48 -7.40(m, 6H), 7.36-7.27(m, 4H), 3.94-3.89(m, 2H), 2.89-2.85(t, 2H).
Embodiment 2
[0058]
[0059] Synthesis of 1-(3-(4-N-carbazolyl))biphenylisoquinoline (2) Weigh 3.52g (7.86mmol) 1-(3-(9-carbazole)biphenyl)- 3,4-dihydroisoquinoline (1) was dissolved in 20mL of 1,3,5-trimethylbenzene, light yellow solution, after argon gas was passed in for 30 minutes, 0.45g of 10% Pd / C was added, and the temperature was raised to 190°C. Reacted for 5h, filtered, washed with dichloromethane, evaporated the solvent, and passed through the column with ethyl acetate:dichloromethane (1:9) to obtain a white solid (3.09g, 88.2%). GC-MS: m / z, 445.2, [M-1] + . 1 H NMR (300MHz, CDCl 3 )(ppm): 8.68-8.67(m, 1H), 8.22-8.14(m, 3H), 8.04(s, 1H), 7.96-8.83(m, 4H), 7.83-7.58(m, 7H), 7.49- 7.39(m, 4H), 7.32-7.27(m, 2H).
Embodiment 3
[0061]
[0062] Synthesis of 1-(3-(4-N-(3,6-dibromocarbazolyl))biphenylisoquinoline (3) 1-(3-(4-N-carbazolyl))biphenyl Base isoquinoline (2) was dissolved in 20mL of dichloromethane, 1.98g (11.12mmol) N-bromosuccinimide was added, and the reaction was stirred at room temperature for 3h. The solvent was evaporated, and the volume ratio was 2:1 Dichloromethane and petroleum ether were used as eluents, and silica gel column chromatography gave white powder (2.83g, 85.8%). ESI-MS: m / z, 605.0, [M+1] + . 1 H NMR (300MHz, CDCl 3 ), (ppm): 8.67-8.65 (d, 1H), 8.20-8.18 (d, 3H), 8.02 (s, 1H), 7.94-7.88 (m, 3H), 7.85-7.62 (m, 4H), 7.58 -7.50(m, 5H), 7.32-7.26(m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com