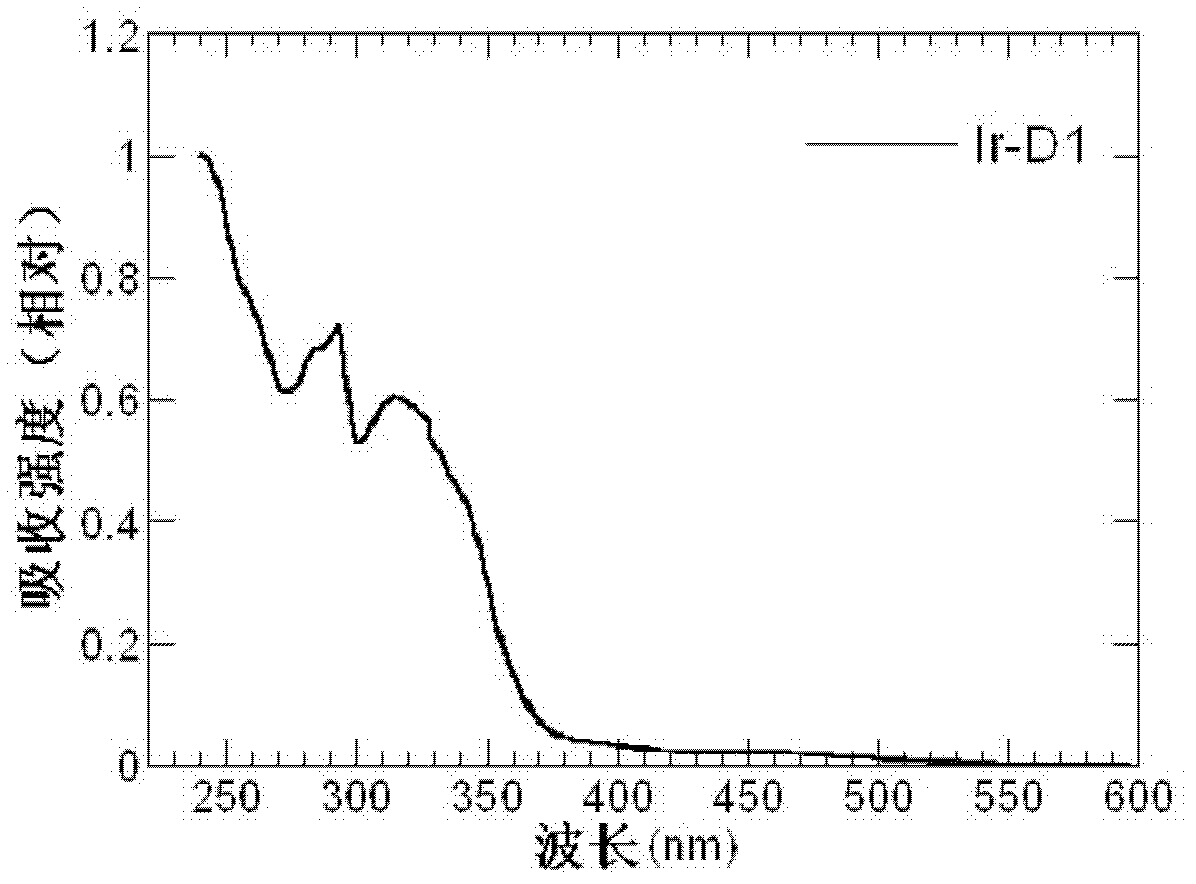
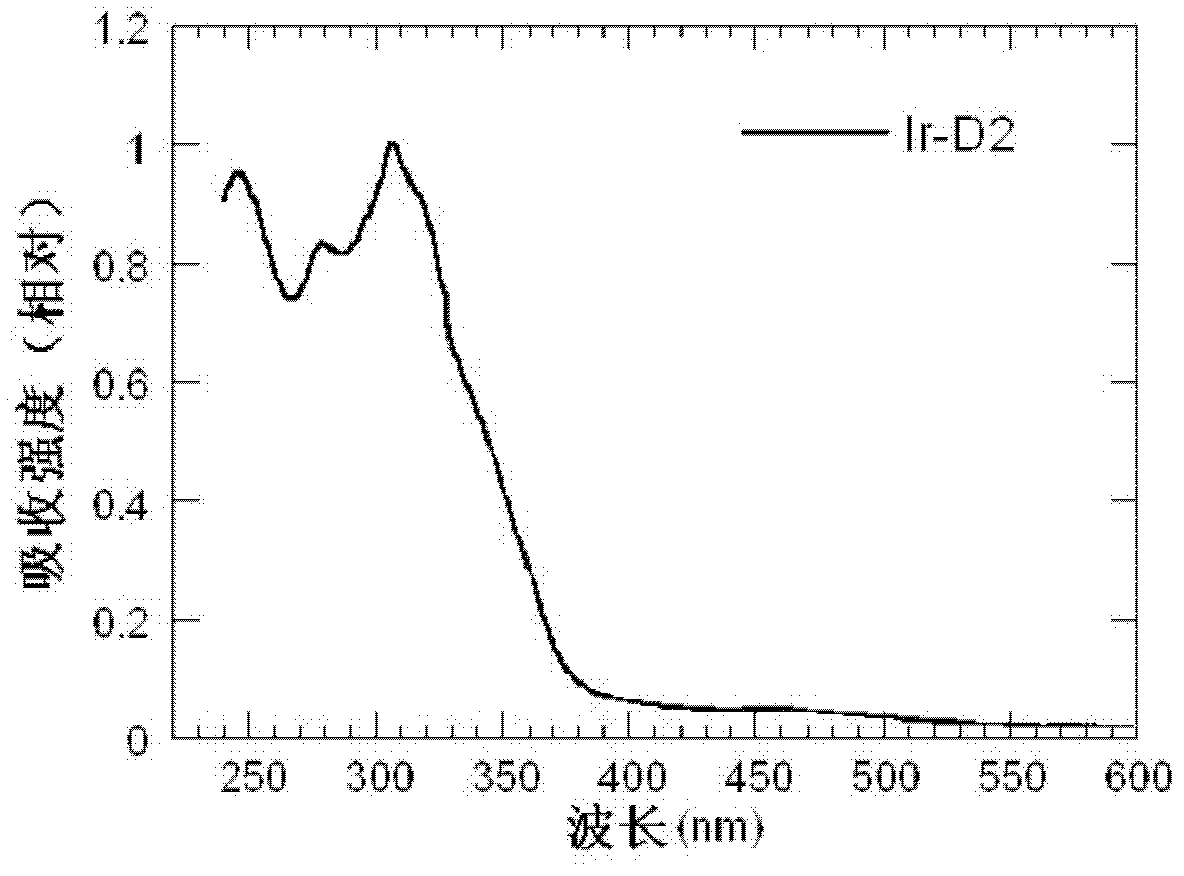
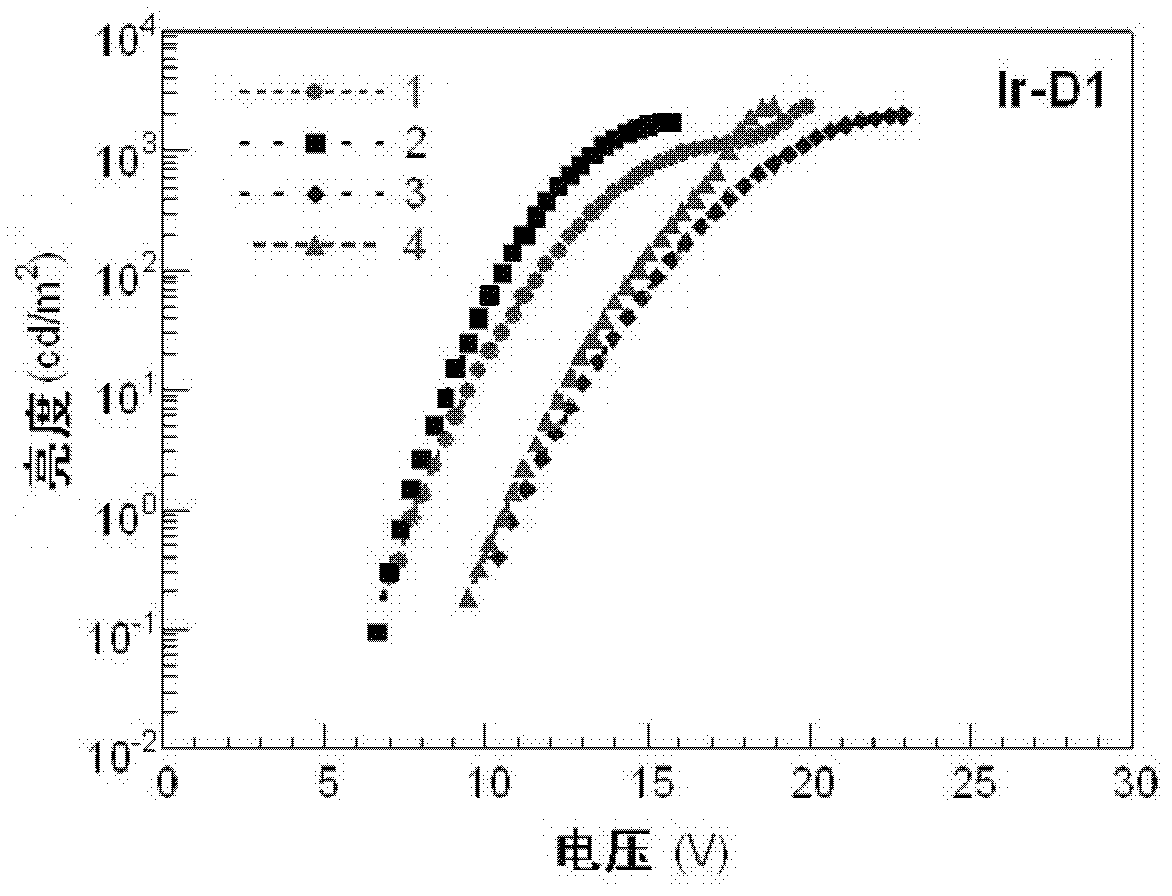
Dendritic iridium coordination compound with double-carrier transport property, application and prepared organic electrophosphorescent device
A technology of transmission properties and iridium complexes, which is applied in the field of organic electrophosphorescent devices and dendritic iridium complexes, can solve the problems of difficult to obtain red electrophosphorescent materials with color purity, increased synthesis difficulty, and poor color purity. Achieve the effect of good development prospects, short reaction time and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] Synthesis of 1-(3-(9-carbazole)biphenyl)-3,4-dihydroisoquinoline (1) 6.50g (22.7mmol) 1-(3'-bromophenyl)-3,4 - dihydroisoquinoline and 9.22 g (25.0 mmol) N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborinyl)phenyl)carbazole, 12.6mL of 2M sodium carbonate solution, 70ml of toluene, and 35ml of ethanol were put into a 200ml three-necked flask, and Pd(PPh 3 ) 4 0.578g, reflux reaction for 24 hours, extracted with dichloromethane, washed with water several times, dried over anhydrous magnesium sulfate, rotovaped the solvent, passed the column with ethyl acetate:dichloromethane (1:9), and obtained a light yellow solid 4.46 g, yield 83.2%. GC-MS: m / z, 447.2, [M-1] + .1H NMR (300MHz, CDCl 3 ), (ppm): 8.17-8.14 (d, 2H), 7.96 (s, 1H), 7.88-7.85 (m, 2H), 7.81-7.78 (m, 1H), 7.66-7.55 (m, 4H), 7.48 -7.40(m, 6H), 7.36-7.27(m, 4H), 3.94-3.89(m, 2H), 2.89-2.85(t, 2H).
Embodiment 2
[0058]
[0059] Synthesis of 1-(3-(4-N-carbazolyl))biphenylisoquinoline (2) Weigh 3.52g (7.86mmol) 1-(3-(9-carbazole)biphenyl)- 3,4-dihydroisoquinoline (1) was dissolved in 20mL of 1,3,5-trimethylbenzene, light yellow solution, after argon gas was passed in for 30 minutes, 0.45g of 10% Pd / C was added, and the temperature was raised to 190°C. Reacted for 5h, filtered, washed with dichloromethane, evaporated the solvent, and passed through the column with ethyl acetate:dichloromethane (1:9) to obtain a white solid (3.09g, 88.2%). GC-MS: m / z, 445.2, [M-1] + . 1 H NMR (300MHz, CDCl 3 )(ppm): 8.68-8.67(m, 1H), 8.22-8.14(m, 3H), 8.04(s, 1H), 7.96-8.83(m, 4H), 7.83-7.58(m, 7H), 7.49- 7.39(m, 4H), 7.32-7.27(m, 2H).
Embodiment 3
[0061]
[0062] Synthesis of 1-(3-(4-N-(3,6-dibromocarbazolyl))biphenylisoquinoline (3) 1-(3-(4-N-carbazolyl))biphenyl Base isoquinoline (2) was dissolved in 20mL of dichloromethane, 1.98g (11.12mmol) N-bromosuccinimide was added, and the reaction was stirred at room temperature for 3h. The solvent was evaporated, and the volume ratio was 2:1 Dichloromethane and petroleum ether were used as eluents, and silica gel column chromatography gave white powder (2.83g, 85.8%). ESI-MS: m / z, 605.0, [M+1] + . 1 H NMR (300MHz, CDCl 3 ), (ppm): 8.67-8.65 (d, 1H), 8.20-8.18 (d, 3H), 8.02 (s, 1H), 7.94-7.88 (m, 3H), 7.85-7.62 (m, 4H), 7.58 -7.50(m, 5H), 7.32-7.26(m, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com