Kangerling granule quality control method
A quality control method, the technology of Kangerling, which is applied in the field of quality control of Kangerling granules, can solve the problems of insufficient quality control methods and no qualitative detection methods for Acanthopanax
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
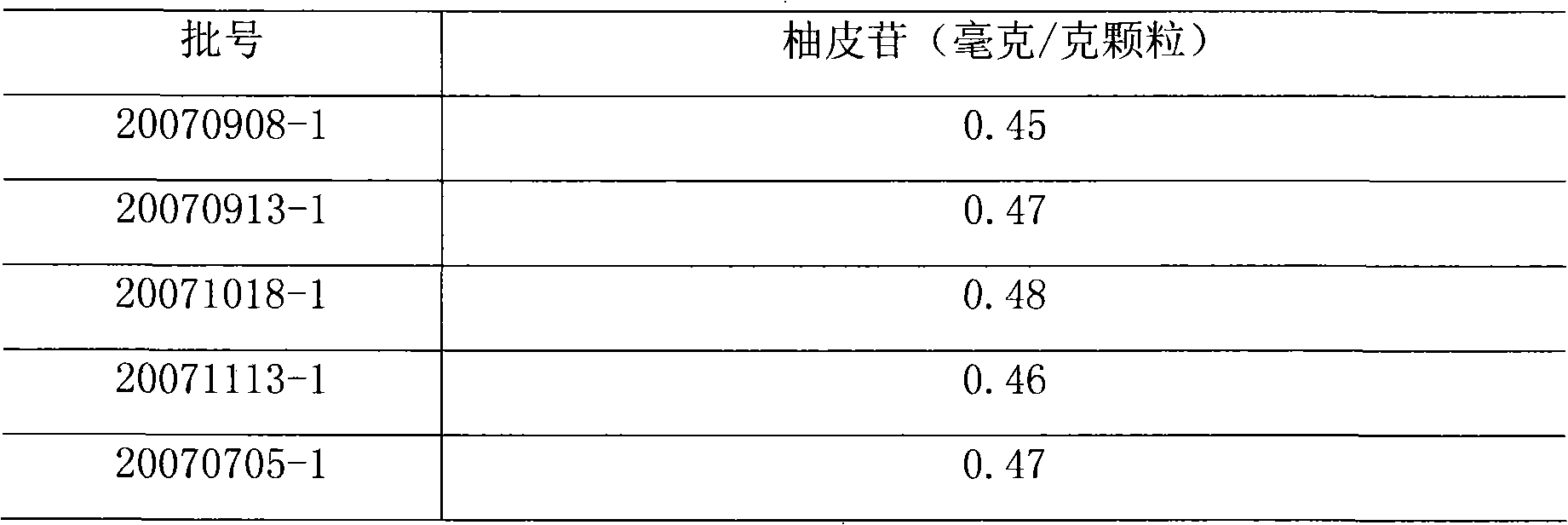
[0026] Five batches of Kangerling granules were randomly selected (batch numbers: 20070908-1, 20070913-1, 20071018-1, 20071113-1 and 20070705-1), and this method was repeated for 5 batches of Kangerling granules.
[0027] Take this product and grind it finely, take 20g of powder, add 100ml of absolute ethanol, ultrasonically treat for 30 minutes, filter, evaporate the filtrate to dryness, add 10ml of water to the residue to dissolve, put it in a separatory funnel, and extract it twice with chloroform, each time 15ml, combined chloroform solution, evaporated to dryness, and dissolved the residue in 1ml of methanol as the test solution. Take isoferidin reference substance and add methanol to make a solution containing 0.2mg per 1ml, as the reference substance solution. Test according to thin-layer chromatography (Appendix VIB), draw 15 microliters of the above-mentioned need testing solution, 4 microliters of reference substance solution, point respectively on the same silica ge...
experiment example 2
[0030] Randomly select Kangerling granules of 5 batch numbers (batch numbers are: 20070908-1, 20070913-1, 20071018-1, 20071113-1 and 20070705-1), and the contents of Kangerling granules are subjected to high performance liquid chromatography ( Chinese Pharmacopoeia 2005 edition an appendix VI D) Determination
[0031] [Check] should meet the relevant regulations under the granules (Appendix I C of Chinese Pharmacopoeia 2005 edition).
[0032] 【Content Determination】Determine according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2005 Edition).
[0033] Chromatographic conditions and system suitability test used octadecylsilane bonded silica gel as filler; acetonitrile-water-phosphoric acid (22:78:0.1) as mobile phase; detection wavelength was 283nm. The number of theoretical plates should not be less than 3000 based on the naringin peak.
[0034] Preparation of Reference Substance Solution Accurately weigh an appropriate amount of naringi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com