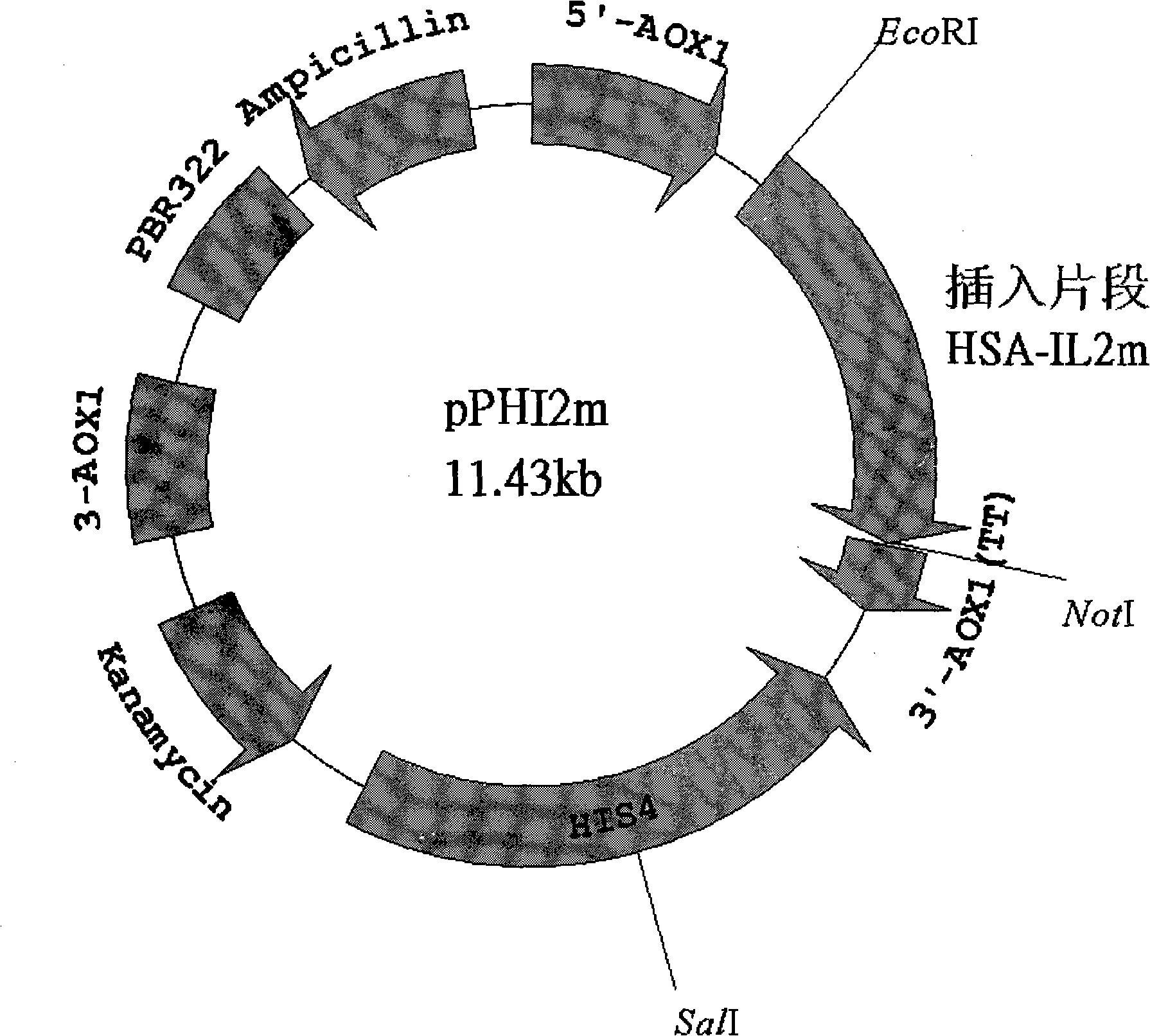
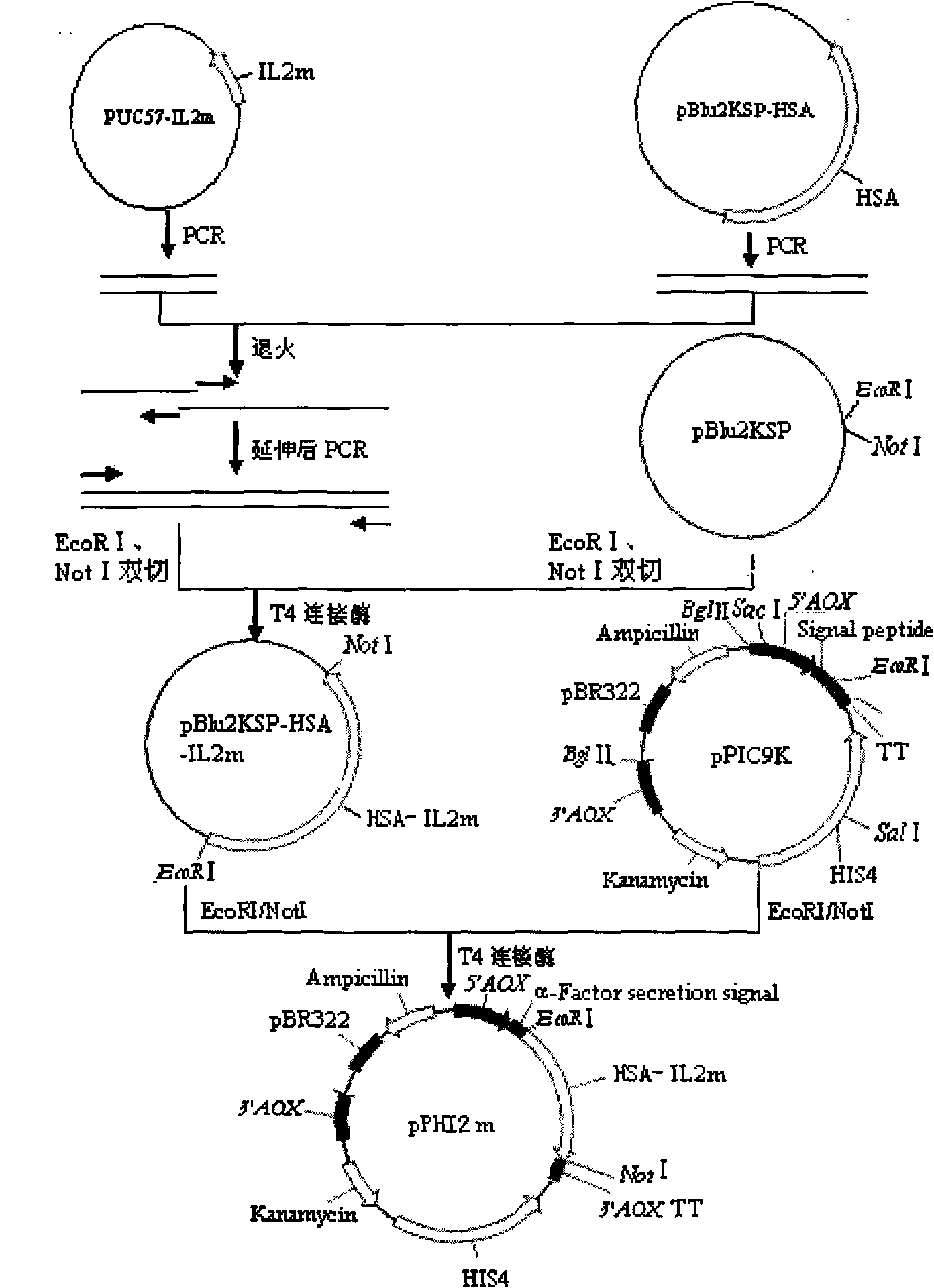
Fused protein of mutant human interleukin-2 and human serum albumin, and preparation thereof
A human serum albumin, human interleukin technology, applied in the field of long-acting fusion protein drugs, can solve the problems of increasing patient pain, treatment costs, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Cloning of IL2m cDNA
[0028] IL2m cDNA (399bp) was artificially synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd., and cloned into the vector PUC57, the insertion site was Sma I, and the recipient bacteria was E. coli DH5α strain.
Embodiment 2
[0029] Example 2: Cloning of HSA cDNA
[0030] HSA cDNA was amplified from the human fetal liver cDNA library by PCR, and the primers used were:
[0031] P1: 5'-AG GTC GAC GATGCACACAAGAGTGAGGTTGCTC-3'
[0032] P2: 5'-GCC AAGCTT TTATAAGCCTAAGGCAGCTTGACTT-3'
[0033] PCR reaction system: 1.5 μl each of 10 μmol / L P1 and P2 primers, 2.5 mmol / L dNTP 4 μl, 10×pfu Buffer 5 μl, 5 U / μl pfu DNA polymerase 0.5 μl, human fetal liver cDNA library 1 μg, double Make up 50 μl with distilled water. PCR reaction program: pre-denaturation at 95°C for 5 minutes; denaturation at 94°C for 1 minute, annealing at 60°C for 1 minute, extension at 72°C for 3 minutes, 30 cycles; extension at 72°C for 10 minutes.
[0034] The reaction product was analyzed by agarose gel electrophoresis, and the target band appeared in the loading lane, and the 1.8kb target fragment was purified with the PCR Fragment Gel Recovery Kit. The purified target fragment and the vector pBlu2KSP were digested by Sal I and H...
Embodiment 3
[0035] Embodiment 3: Cloning of IL2m cDNA and HSA cDNA fusion gene
[0036] (1) PCR amplification of IL2m cDNA, the primers used are as follows:
[0037] HI1: 5'-CAAGCTGCCTTAGGCTTAGCACCTACTTCAAGTTCTAC-3'
[0038] HI2: 5'-A GCGGCC GCTTAAGTCAGTGTTGAGATGATGC-3'
[0039] PCR reaction system: 1.5 μl each of 10 μmol / L HI1 and HI2 primers, 2.5 mmol / L dNTP 4 μl, 10×pfu Buffer 5 μl, 5 U / μl pfu DNA polymerase 0.5 μl, plasmid template PUC57-IL2m 1ng, add double Make up 50 μl with distilled water. PCR reaction program: pre-denaturation at 95°C for 5 minutes; denaturation at 94°C for 1 minute, annealing at 65°C for 1 minute, extension at 72°C for 30 seconds, 30 cycles; extension at 72°C for 10 minutes.
[0040] (2) PCR amplification of HSA cDNA, the primers used are as follows:
[0041] HI3: 5'-G GAATTC AAAAGAGATGCACACAAGAGTGAGGT-3'
[0042] HI4: 5'-GTAGAACTTGAAGTAGGTGCTAAGCCTAAGGCAGCTTG-3'
[0043] PCR reaction system: 1.5 μl each of 10 μmol / L HI 3 and HI 4 primers, 2.5 mmol / L d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap