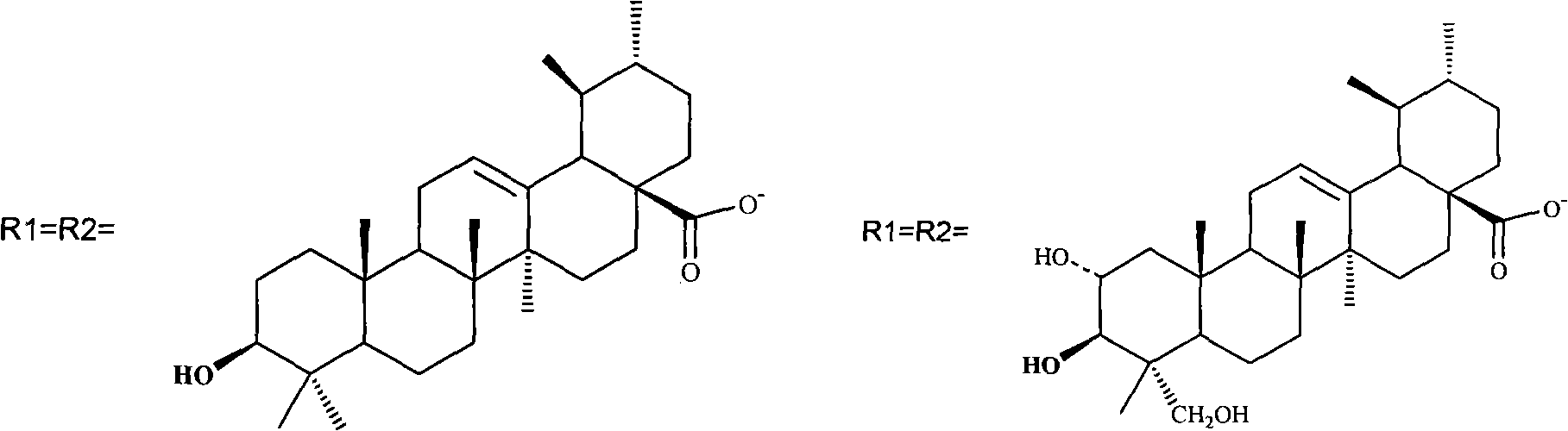Platinum salts of organic acids, preparation thereof and applications in preparation of anticancer drugs
A technology of organic acid and salt products, applied in the field of medicine, can solve problems such as no reports on anti-tumor research of platinum salt derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthesis of embodiment 1, platinum ursolic acid I
[0043] 1. Weigh trans-cyclohexanediamine (549mg, 4.82mMol) and potassium chloroplatinite (2.00g, 4.82mMol) and dissolve them in 100-200ml water at room temperature and stir in the dark, and react for 5-10 hours to obtain The yellow solid was filtered and dried under reduced pressure to obtain 1.138g [Pt(C 6 h 14 N 2 ) Cl 2 ], yield 72.56%.
[0044] 2. Set [Pt(C 6 h 14 N 2 ) Cl 2 ], (1.1g, 2.9mMol) was suspended in 40-100ml of water, silver nitrate (0.986g, 5.8mMol) was added, stirred at room temperature for 112-24 days, and the formed AgCl precipitate was filtered.
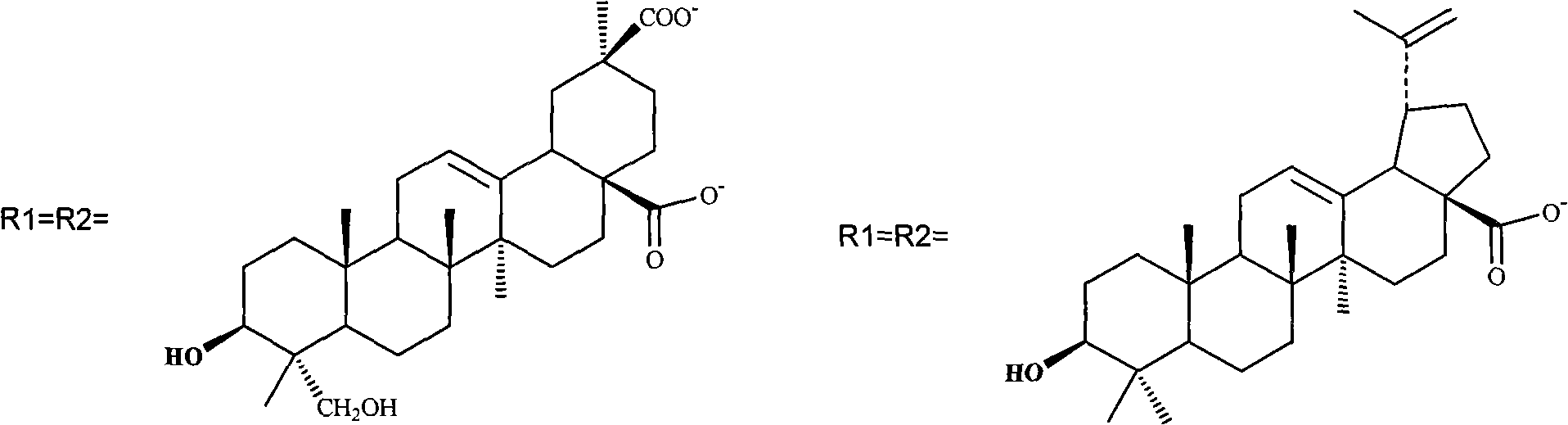
[0045] 3. Mix ursolic acid (2.668g, 5.8mMol) with NaOH (232mg, 5.8mMol), add to the solution just obtained, stir at room temperature, keep away from light, overnight. 2.6 g of platinum ursolic acid I product was obtained. See above for chemical structure.
Embodiment 2
[0046] Embodiment 2, the synthesis of platinum ursolic acid II
[0047] Measure ammoniacal liquor 25ml (100mg, 5.88mMol) and potassium chloroplatinite (2.00g, 4.82mMol) and dissolve in 160ml water at room temperature and stir in the dark, react for 10 hours, then add silver nitrate (0.986g, 5.8mMol ), stirred at room temperature for one day, and the formed AgCl precipitate was removed by filtration. A mixture of ursolic acid (2.675 g, 5.8 mMol) and NaOH (230 mg, 5.8 mMol) was added to the filtrate, stirred at room temperature, protected from light, overnight. 2.3 g of platinum ursolic acid II product was obtained. See above for chemical structure.
Embodiment 3
[0048] The synthesis of embodiment 3, platinum betulinate I
[0049] 1. Weigh trans-cyclohexanediamine (549mg, 4.82mMol) and potassium chloroplatinite (2.00g, 4.82mMol) and dissolve them in 150ml of water at room temperature and stir in the dark, react for 10 hours, produce a precipitate, filter, Dry under reduced pressure to obtain 1.24g [Pt(C 6 h 14 N 2 ) Cl 2 ].
[0050] 2. Take [Pt(C 6 h 14 N 2 ) Cl 2 ], (1.1g, 2.9mMol) was suspended in 60ml of water, silver nitrate (0.99g, 5.8mMol) was added, stirred at room temperature for 24 hours, and the formed AgCl precipitate was removed by filtration. Added to the filtrate, mixed betulinic acid (2.65g, 5.8mMol) and NaOH (230mg, 5.8mMol), added to the solution just obtained, stirred at room temperature, protected from light, overnight. 2.7 g of platinum betulinate I product was obtained. See above for chemical structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com