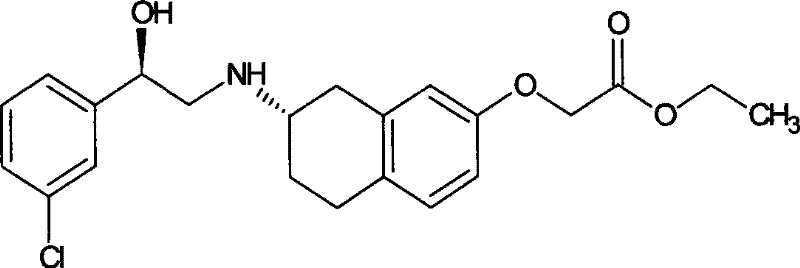
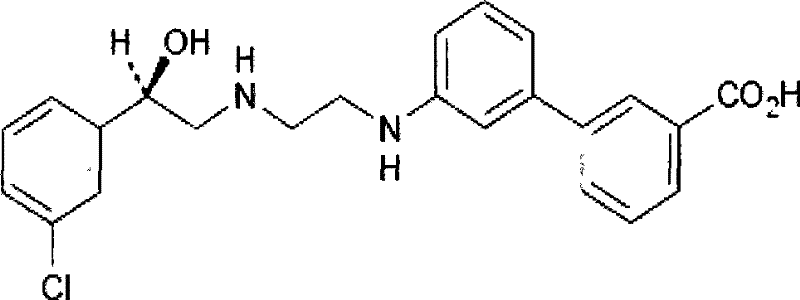
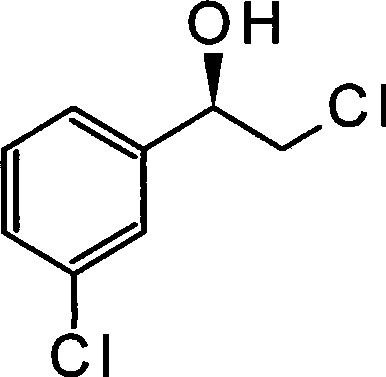
Method for preparing (R)-2-chlorin-1-(3-chlorphenyl) ethanol by microorganism catalysis
A catalytic preparation and microbial technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve problems such as harsh reaction conditions, pollution, complex enzyme systems, etc., and achieve mild reaction conditions, high optical purity, high The effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Cultivation and screening of microbial strains
[0039] Slope culture: the medium is 100mL wort (containing 10g of malt powder), 2g of agar, pH 6.0, sterilized at 121°C for 20 minutes, cooled and inoculated after sterilization, and the strains are various microbial strains shown in Table 1, 28 Cultivate for 2 days at ℃, and use as slant activated seeds.
[0040] Seed cultivation and fermentation: Yeast extract 10g / L, peptone 20g / L, glucose 20g / L, pH6.5, liquid volume is 250mL triangular bottle 50mL, sterilized at 120°C for 20 minutes, cooled and inoculated with slant seeds after sterilization, 180r / min shaker, culture at 28°C for 24-48 hours, as seeds or fermentation broth.
[0041] The amount of wet bacteria in the fermentation broth is 1.6g / 100mL, centrifuge for 10 minutes (8,000 rpm) to collect the bacteria, wash twice with potassium phosphate buffer (0.2M, pH6.5), transfer the bacteria to 5mL In the same buffer solution containing glucose (0.5M), make the cell con...
Embodiment 2
[0048] Effect of different carbon sources on transformation
[0049] Alcaligenes faecalis (Alcaligenes faecalis) ATCC 15554 was used as the transformed strain, and the strain was cultivated according to the method in Example 1, and different carbon sources were added in Example 1 instead of glucose. Inoculate the strains from the slant into 5ml liquid medium, pre-cultivate on an air shaker at 28°C, 180r / min for 24h, then transfer to 50ml medium for expansion for 24h, and centrifuge at 12000r / min for 8min at 4°C. The reaction conditions and collection method are the same as in Example 1. The results are shown in Table 2.
[0050] Table 2 Effect of different carbon sources on transformation
[0051]
[0052] It can be seen from the results in Table 2 that the sample e.e. value can reach more than 99%, and the repeatability of each parallel sample is good. Considering the conversion rate, it was determined that corn steep liquor was used as the medium carbon source in the e...
Embodiment 3
[0054] Effect of Different Nitrogen Sources on Transformation
[0055] Alcaligenes faecalis (Alcaligenes faecalis) ATCC 15554 strain was cultured according to the method of Example 1, in which different nitrogen sources (shown in Table 3) were added to the glucose medium in Example 1. Inoculate the strains from the slant into 5ml liquid medium, pre-cultivate on a shaker at 180r / min at 28°C for 24h, then transfer to 50ml medium for expansion for 24h, and centrifuge at 12000r / min for 8min at 4°C. The reaction conditions and collection method are the same as in Example 1. The results are shown in Table 3.
[0056] Table 3 Effect of different nitrogen sources on transformation
[0057]
[0058] As can be seen from the results in Table 3: with (NH 4 ) 2 SO 4 The conversion of the sample as a nitrogen source was high (80.22%) with an e.e. value of 99.79%, but there was considerable variation between levels. Considering comprehensively, yeast extract plus common peptone was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com