Fluorochrome for marking oligonucleotide and protein, method for preparing same and use
A fluorescent dye and protein labeling technology, applied in the field of oligonucleotide and protein labeled fluorescent dyes and their preparation, can solve the problems of ineffective application, affecting the effect of electrophoretic separation, difference in energy absorption and emission capabilities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation reaction principle of fluorescent dye of the present invention is as follows:
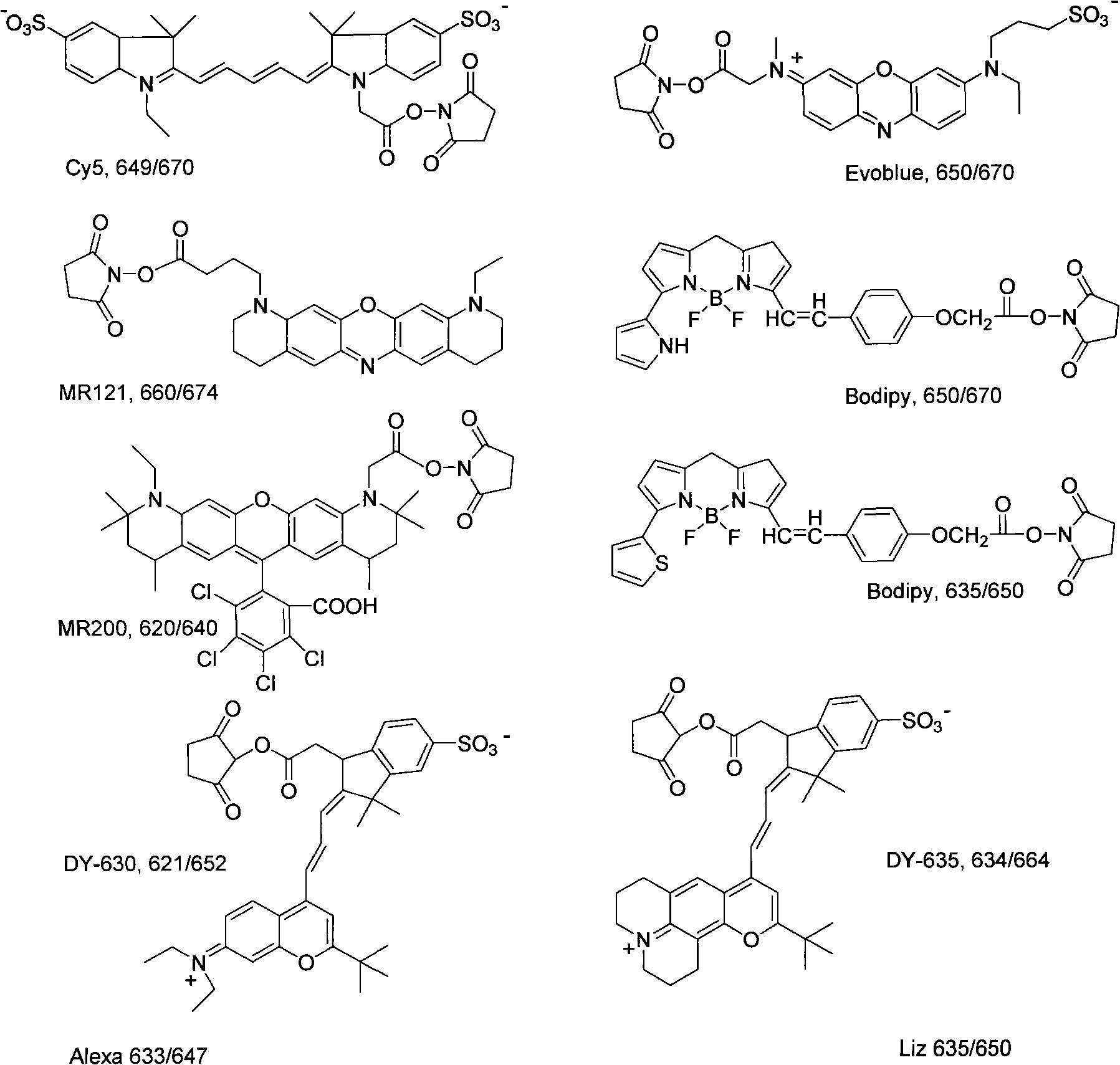
[0026] Synthesis principle of infrared wavelength fluorescent dyes synthesized by energy conversion method
[0027]
[0028] Infrared wavelength fluorescent molecular synthesis mechanism:
[0029]
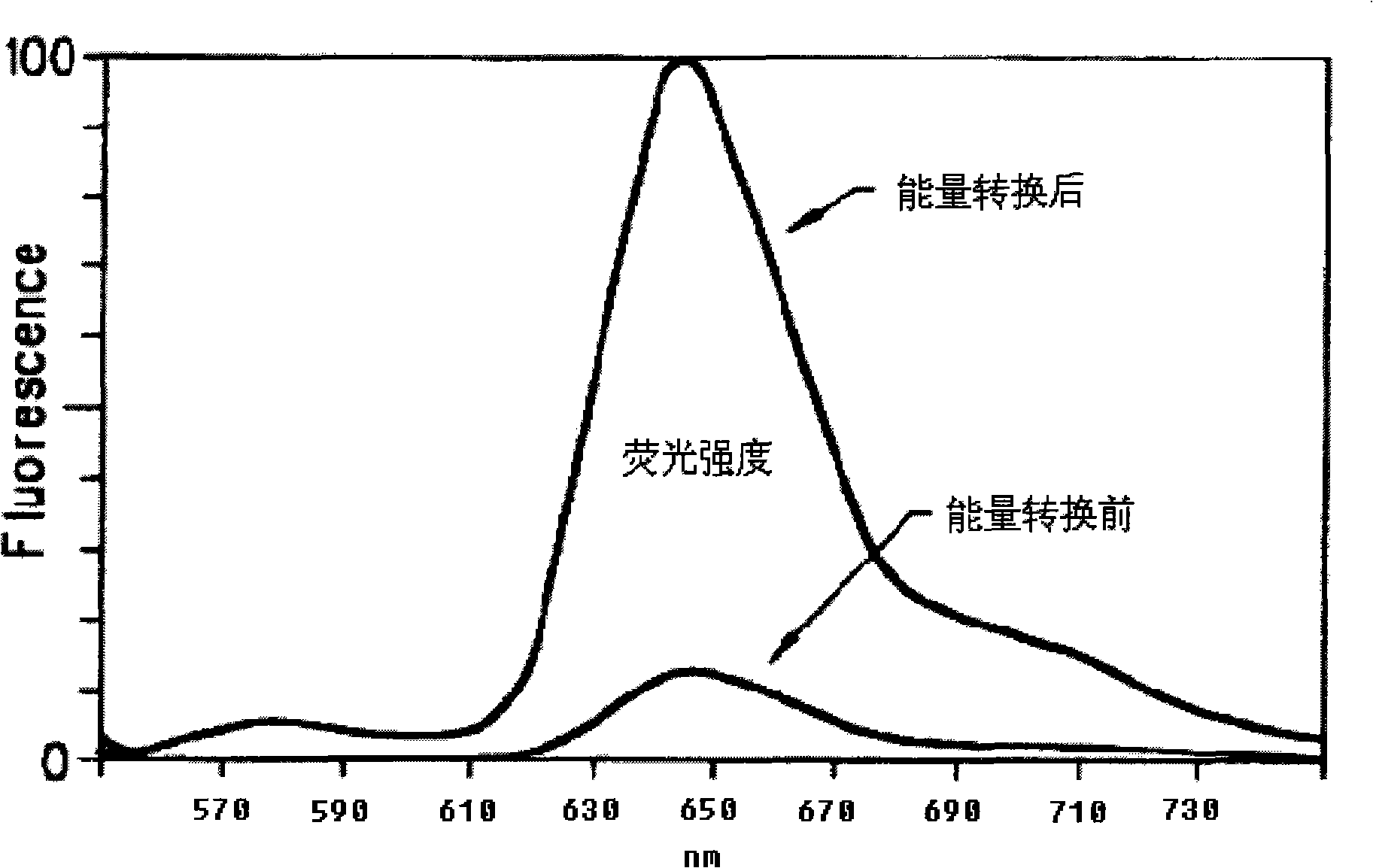
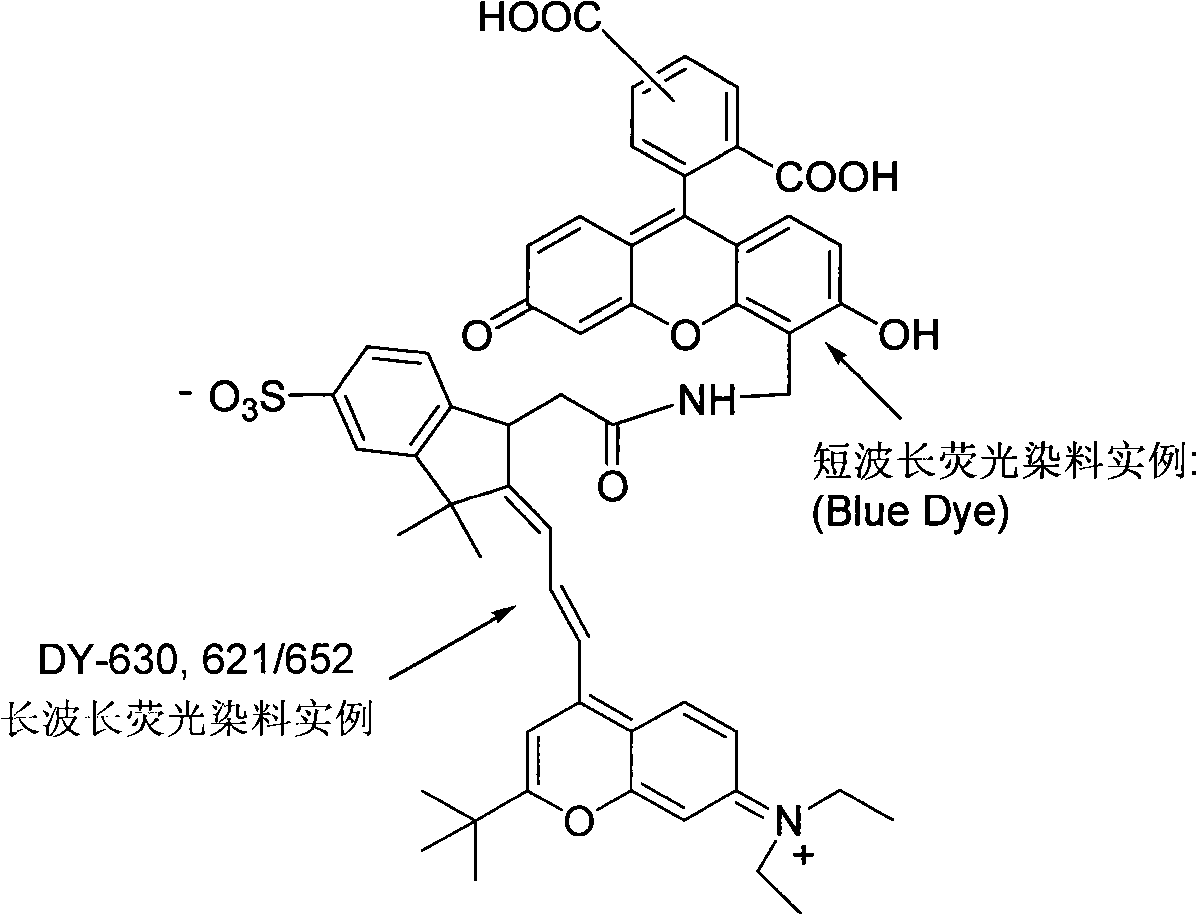
[0030] The long-wavelength fluorescent dye DY-630 succinate and aminated short-wavelength fluorescein are dissolved in dimethylformamide, and under the catalysis of triethylamine, they are connected to form a new one that can efficiently absorb energy at a wavelength of 480-520nm And release fluorescent molecules with strong fluorescence at 650nm wavelength
Embodiment 1
[0032] Aminated short-wavelength fluorescein with a wavelength of 460-520nm, which is a single isomer, and 0.1 mmole of long-wavelength fluorescent dye DY-630 succinate were dissolved in 100 μl of dimethylformamide at a molar ratio of 1:1. 10 μl of triethylamine was added and under its catalysis, the reaction was carried out at room temperature. After 1 hour of reaction, 200 μl of weakly alkaline sodium carbonate solution with pH 7-10 was added and mixed. The reaction was further carried out at 60° C. for 1 hour. After the reaction finishes, remove the precipitate by high-speed centrifugation, after the supernatant is concentrated under reduced pressure (heatable, but should be lower than 60°C), dissolve the methanol in 10ml 5% solution in dichloromethane, and use 1000ml5-30% ( The concentration gradient solution of methanol in dichloromethane (volume ratio concentration) is separated by silica gel column chromatography to remove reaction by-products and unreacted compounds, c...
Embodiment 2
[0034] Aminated short-wavelength fluorescein, which is a mixture of isomers, and 0.1 mmole of long-wavelength fluorescent dye DY-630 succinate were dissolved in 200 μl of dimethylformamide at a molar ratio of 20:1. Add 20 μl of triethylamine under its catalysis, react at below 60° C., after 12 hours of reaction, add 500 μl of pH 7-10 weak alkaline sodium carbonate aqueous solution and mix. Then react at 60°C for 24 hours. After the reaction finishes, high-speed centrifugation removes the precipitate, and the supernatant is concentrated under reduced pressure (heatable, but should be lower than 60°C), and then dissolved in 10ml of 5% methanol in dichloromethane, and mixed with 1000ml of 5-30% The concentration gradient solution of methanol in dichloromethane (volume specific concentration) is separated by silica gel column chromatography, removes reaction by-products and unreacted compounds, collects the last colored fluorescent component, and concentrates under reduced pressur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com