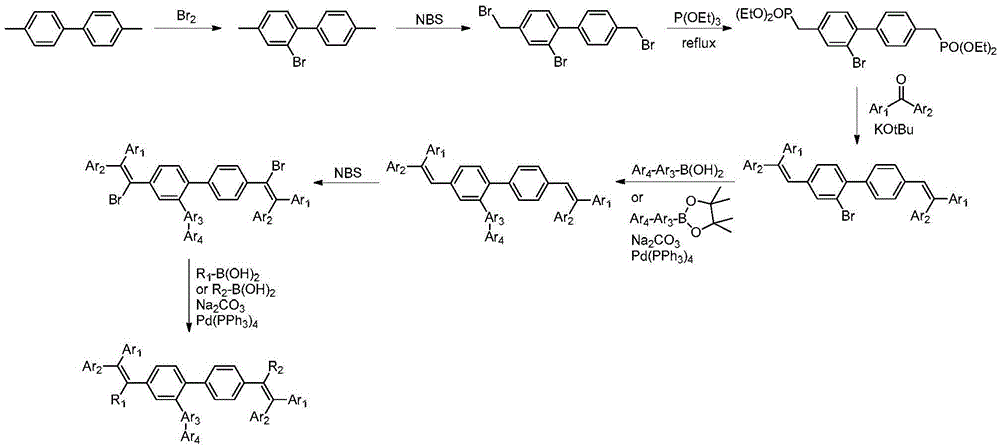
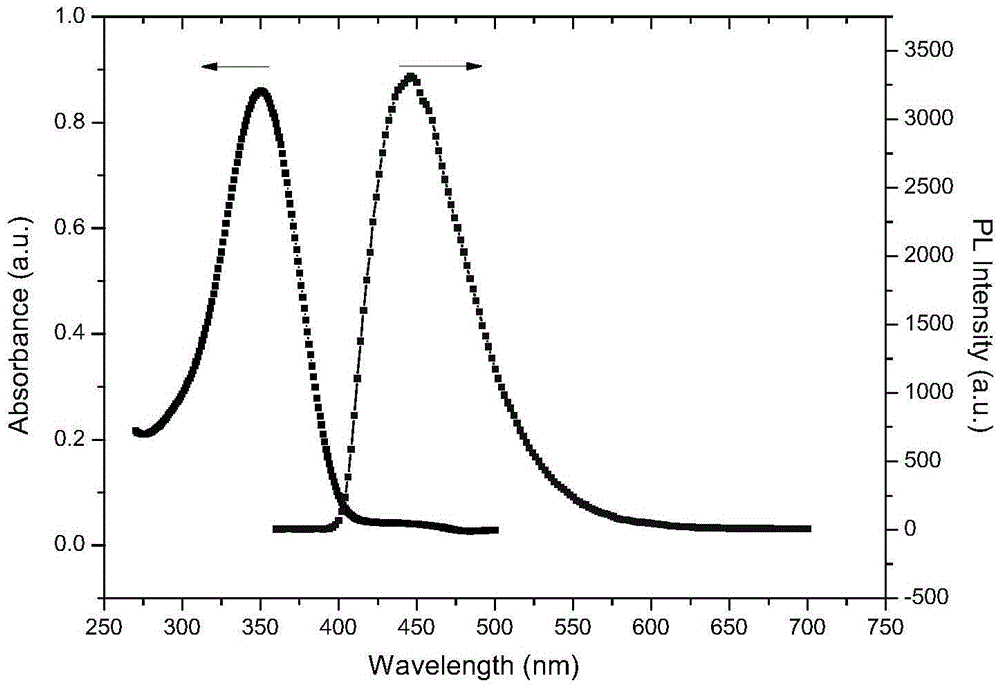
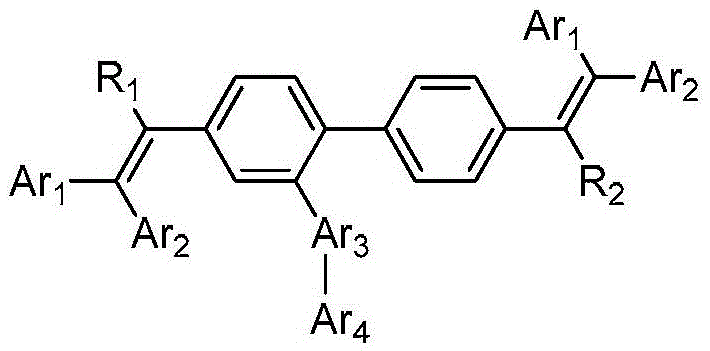
Series of fluorescent OLED materials
A C6-C60, unsubstituted technology used in the field of organic electroluminescence display
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1, the preparation of compound formula 17
[0089]
[0090] Step 1: Preparation of 2-bromo-4,4’-dimethylbiphenyl
[0091]
[0092] Dissolve 36.5g (0.2mol) of 4,4'-dimethylbiphenyl in 360ml of anhydrous dichloromethane, add 1 grain of iodine at room temperature, stir for 1 hour, slowly add 35.2g (0.22mol) of bromine dropwise The solution dissolved in dichloromethane was stirred and reacted for 24 hours, 120ml of saturated aqueous sodium bisulfite solution was added, stirred and reacted for 1 hour, the organic phase was separated, the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure , separated and purified by silica gel column, and concentrated to dryness under reduced pressure to obtain 48 g of a colorless oil, with a yield of 92%.
[0093] The second step: the preparation of 2-bromo-4,4'-dibromomethylenebiphenyl
[0094]
[0095] Dissolve 46g (0.176mol) of 2-bromo-...
Embodiment 2
[0113] Embodiment 2, the preparation of compound formula 55
[0114]
[0115] The first step: the preparation of compound formula 20
[0116]
[0117] The synthesis operation of this step refers to the fifth step of Example 1, using 10g (16.9mmol) 2-bromo-4,4'-bis(2,2-stilbene)biphenyl and 8.5g (20mmol) 3,5- Diphenyl-4-(4-(boronic acid pinacol ester) phenyl)-4H-1,2,4-triazole was subjected to a coupling reaction, separated and purified on a silica gel column to obtain 5.8 g of a yellow solid, Yield 80%.
[0118] The second step: intermediate 4-(4",5'-bis(1-bromo-2,2-distyryl)-[1,1':2',1"-triphenyl]-4- The preparation of -3,5-diphenyl-4H-1,2,4-triazole
[0119]
[0120] The compound formula 20 of 5g (6.2mmol) is mixed with 250ml carbon tetrachloride, adds the free radical initiator AIBN of the NBS of 2.4g (13.6mmol) and 10mg (0.06mmol), is warming up to reflux stirring reaction 24 hours, is cooled to room temperature , add 100ml of water to dilute, heat up to reflu...
Embodiment 3
[0132] Embodiment 3, the preparation of compound formula 95
[0133]
[0134] Step 1: Preparation of intermediate 9,9'-((2-bromo-[1,1'-biphenyl]-4,4'-diyl)bis(methylene))bis(9H-fluorene)
[0135]
[0136] Dissolve 20g (37.5mmol) of 2-bromo-4,4'-bis(phosphodiethylesterylmethylene)biphenyl in 100ml of dry tetrahydrofuran, add 13.5g (75mmol) of 9-fluorenone, and 10g (90mmol) of potassium tert-butoxide was added in batches, stirred for 8 hours, heated and refluxed for 4 hours, cooled to room temperature, poured into 500ml of ice water, extracted with ethyl acetate, and the organic phase was anhydrous sodium sulfate After drying and filtering, the filtrate was concentrated to dryness under reduced pressure, separated and purified by silica gel column, and eluted with petroleum ether to obtain 16.6 g of yellow solid with a yield of 75%.
[0137] The second step: the preparation of compound formula 95
[0138]
[0139] 10g (17mmol) of the upper step intermediate, 6.4g (20....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com