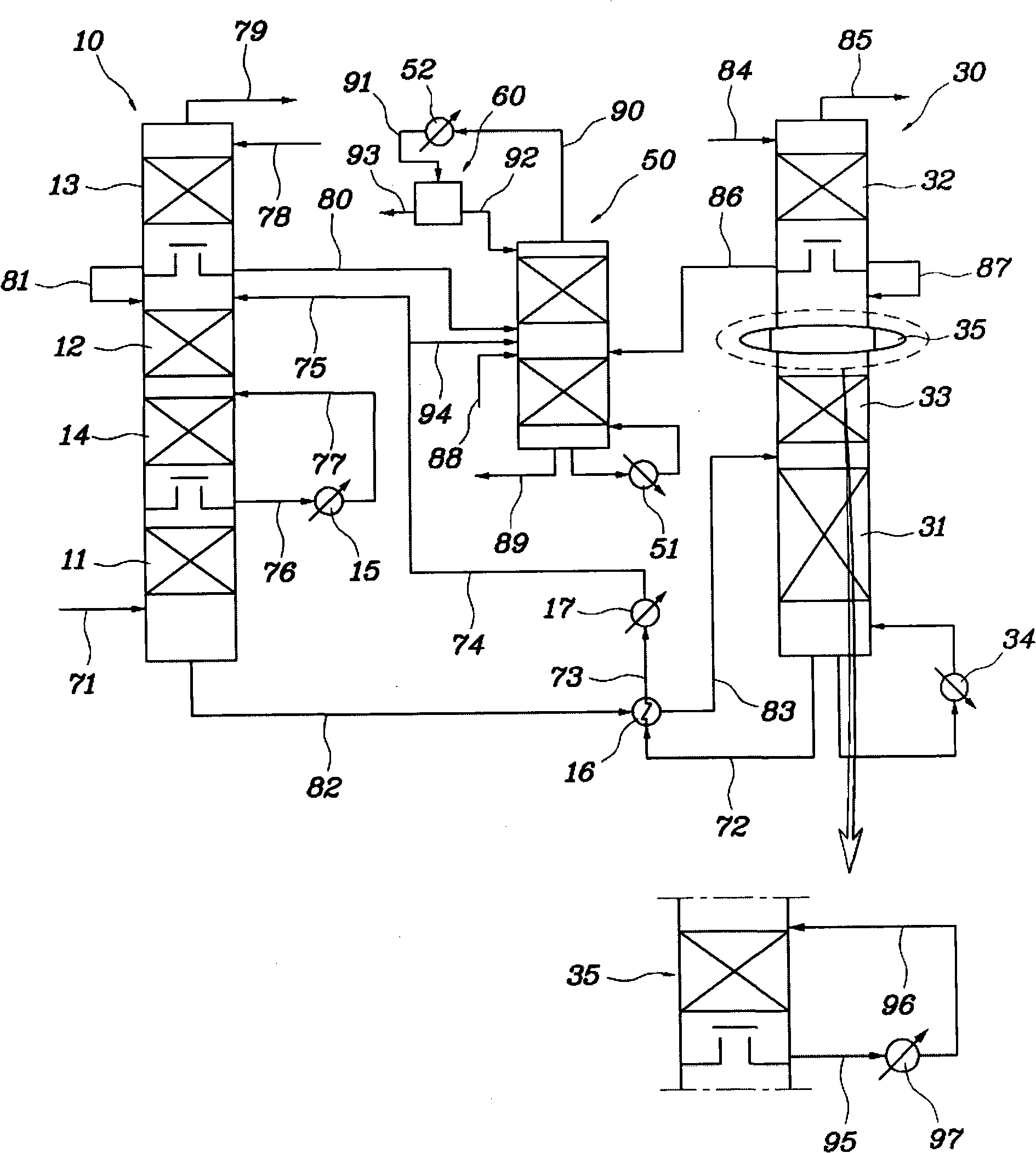
Apparatus and method for recovering carbon dioxide from flue gas using ammonia water
A carbon dioxide and flue gas technology, applied in chemical instruments and methods, separation methods, inorganic chemistry, etc., can solve problems such as temperature rise, deterioration effect, and temperature aggravated ammonia volatilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
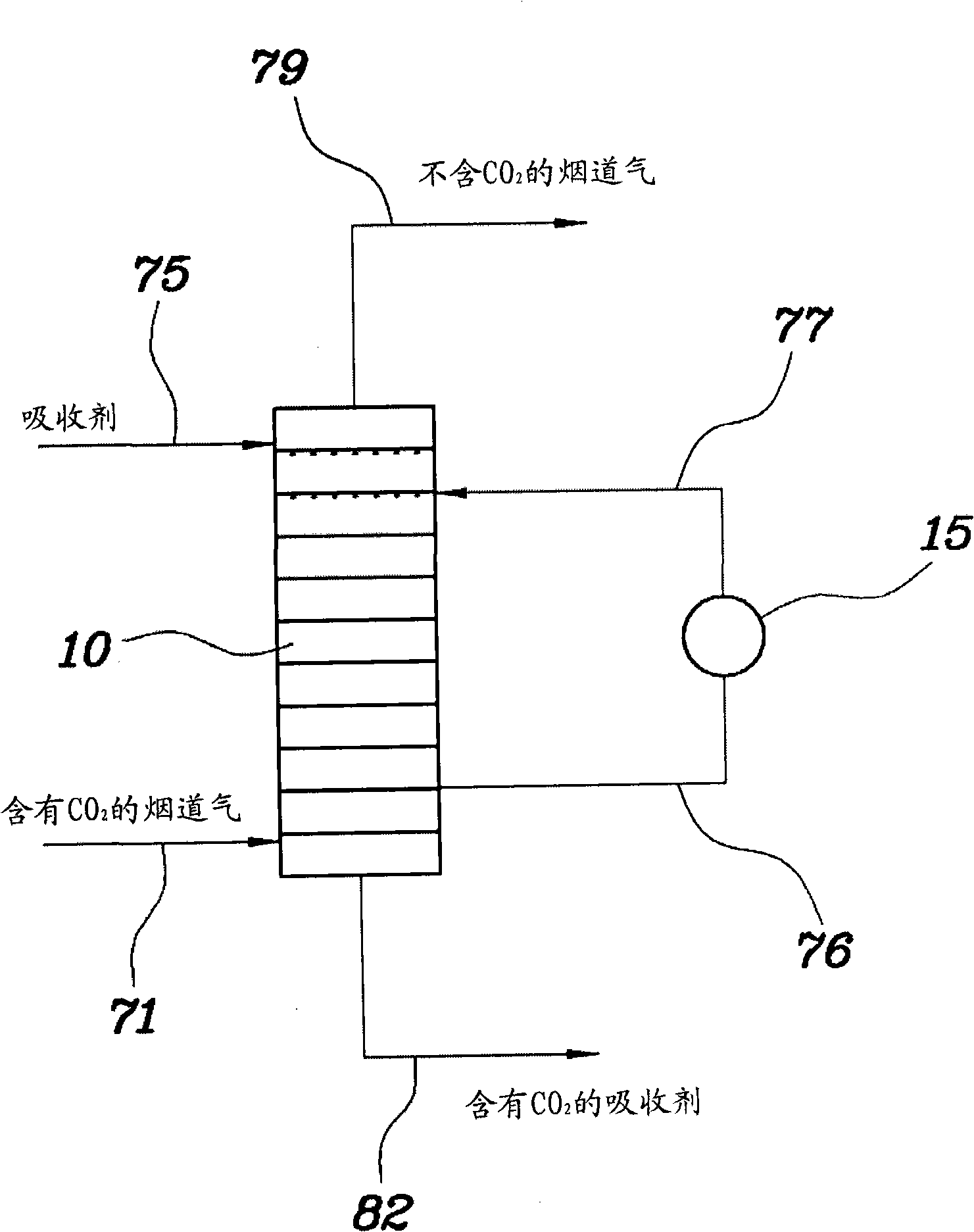
[0049] Adopt the absorption tower with the same structure and size as in the comparative example and the model gas with the same composition, reclaim the absorbent from the fourth column plate from the top of the absorption tower, cool the absorbent to 30 ° C, and then The absorbent is again supplied to the fourth tray from the top of the absorption column, wherein said fourth tray has the highest temperature. In this way, the amount of absorbent recovered is 1 / 6 of the amount initially supplied to the absorption tower.
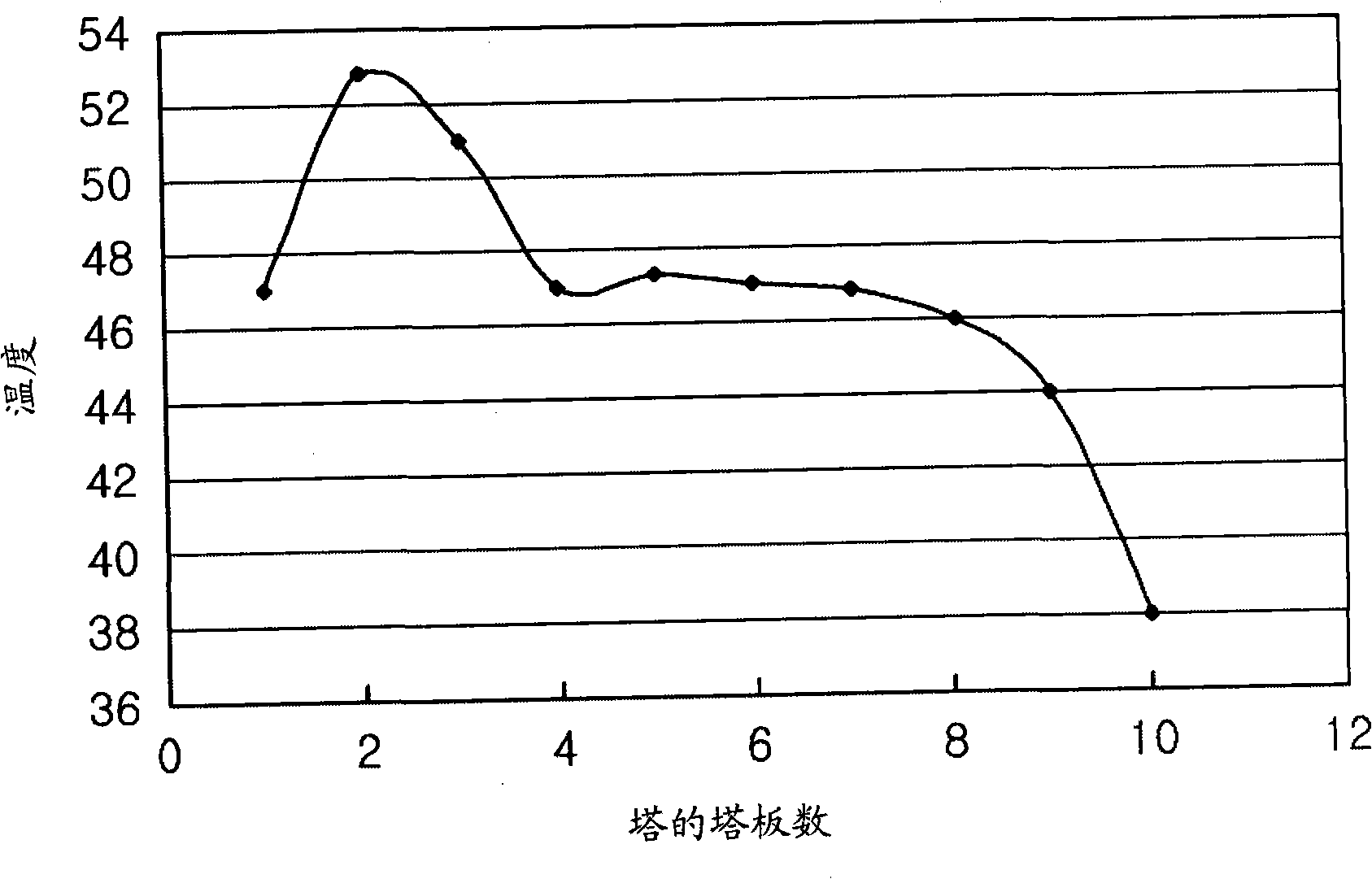
[0050] As a result of Example 1, the temperature distribution inside the absorption tower is shown at Figure 4 middle. The carbon dioxide absorption efficiency was determined to be 88%.
example 2
[0052] Adopt the absorption tower with the same structure and size as in the comparative example and the model gas with the same composition, recover the absorbent from the fourth column plate from the top of the absorption tower, cool the absorbent to 20 ° C, and then The absorbent is again supplied to the fourth tray from the top of the absorption column, wherein said fourth tray has the highest temperature. In this way, the amount of absorbent recovered is 1 / 3 of the amount initially supplied to the absorption tower.
[0053] As a result of Example 2, the temperature distribution inside the absorption tower is shown at Figure 5 middle. The carbon dioxide absorption efficiency was determined to be 91%.
example 3
[0055] Adopt the absorption tower with the same structure and size in the comparative example and the model gas with the same composition, by reclaiming the absorbent from the fourth column tray from the top of the absorption tower, cooling the absorbent to 25°C, and then The absorbent is again supplied to the third tray from the top of the absorption column, wherein said fourth tray has the highest temperature. In this way, the amount of absorbent recovered is 1 / 3 of the amount initially supplied to the absorption tower.
[0056] As a result of Example 3, the temperature distribution inside the absorption tower is shown at Figure 6 middle. The carbon dioxide absorption efficiency was determined to be 91%.
[0057] It is clear from Examples 1 to 3 that part of the high-temperature absorbent is recovered and cooled by a recirculation cooler, and then supplied again to the absorption tower, thus significantly improving the CO2 absorption compared to the CO2 absorption efficie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com