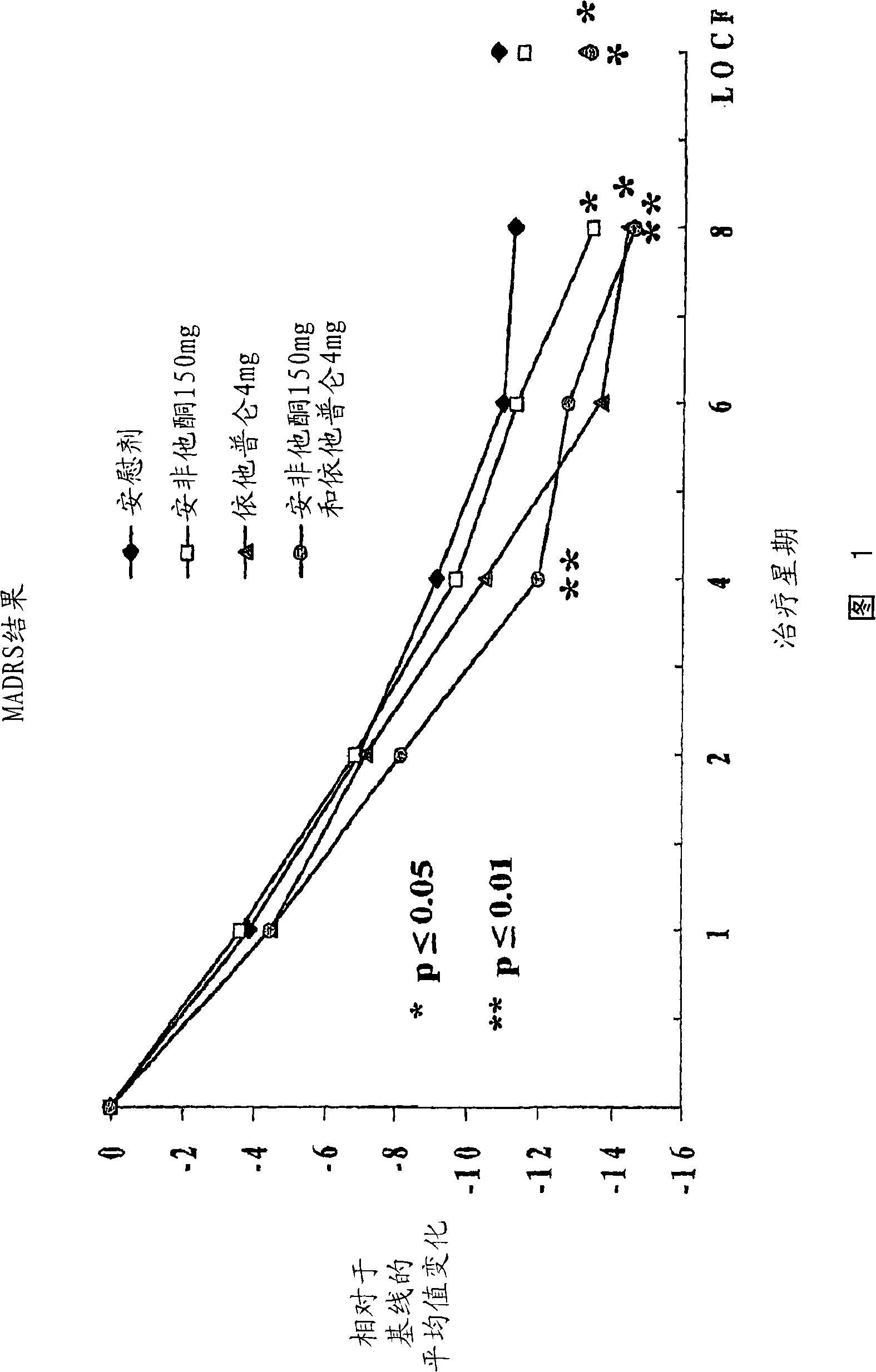
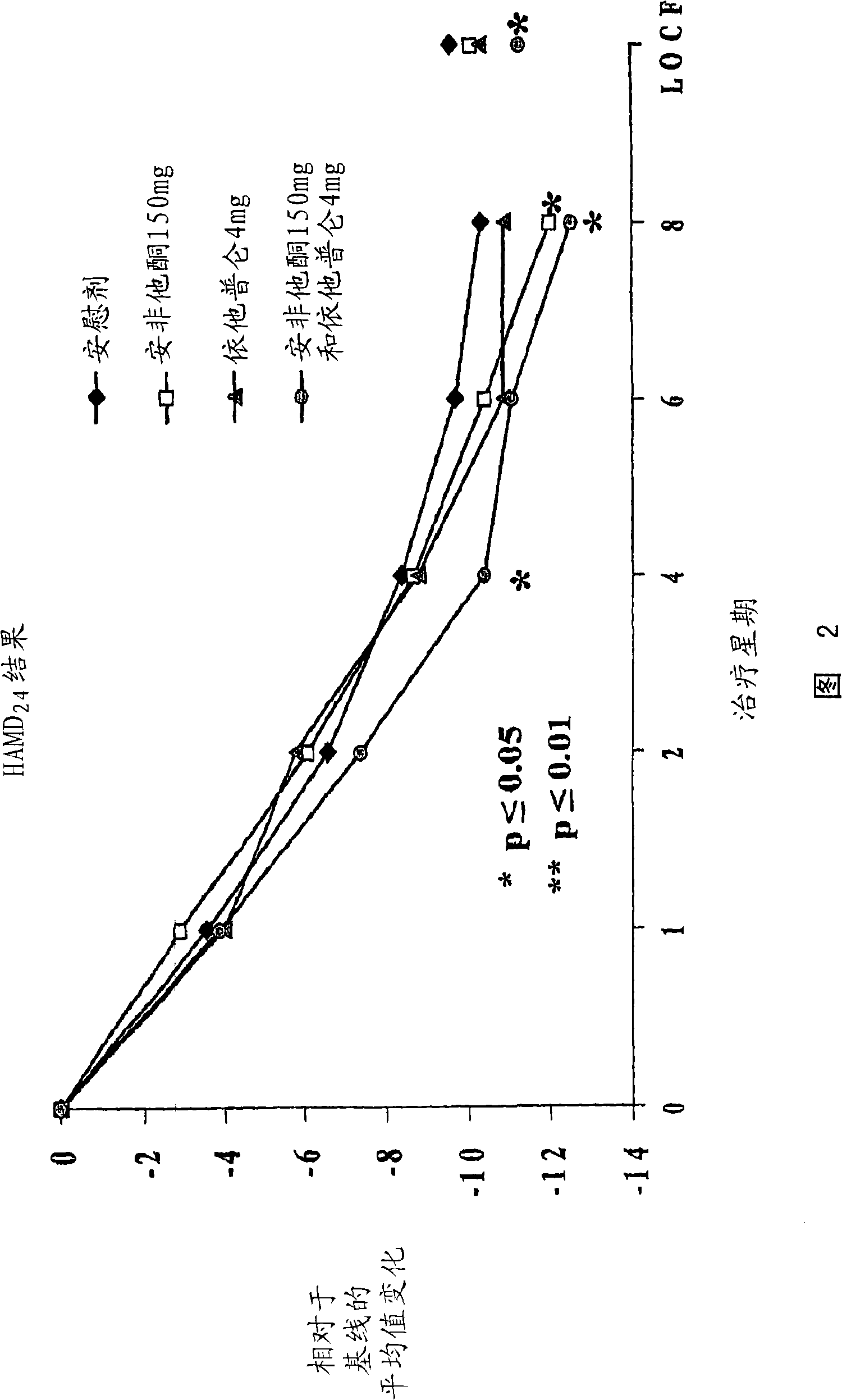
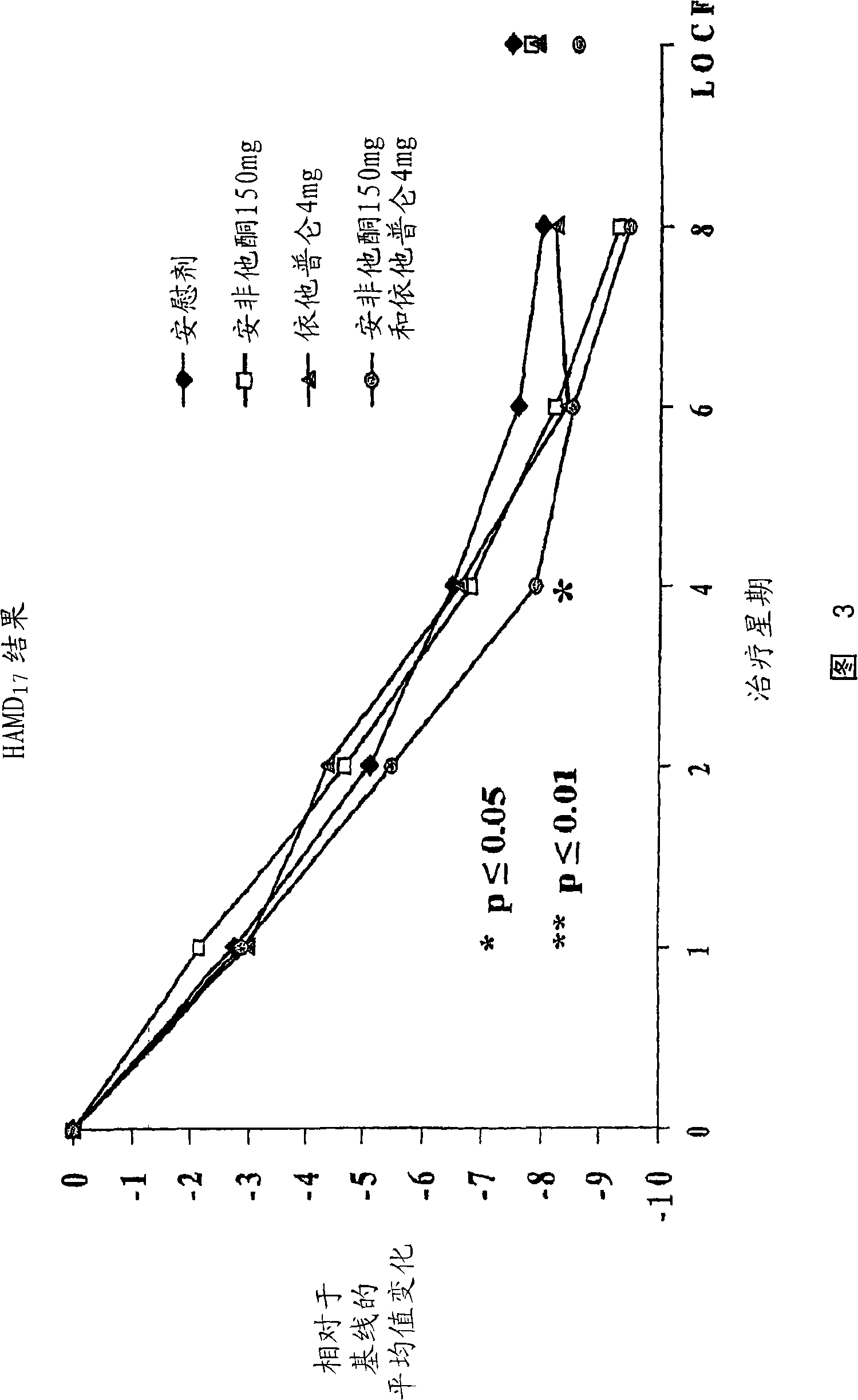
Methods of treating central nervous system disorders with a low dose combination of escitalopram and bupropion
A technology of escitalopram and bupropion, which is applied in the field of treating central nervous system diseases, and can solve the problems that antidepressant treatment cannot produce complete response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Escitalopram core and modified release beads
[0105] Tables 1 and 2 show the formulation ingredients and weight percent ranges used to make escitalopram cores and modified release beads, respectively. Each modified release bead was an escitalopram core bead coated with a modified release coating.
[0106] Table 1: Escitalopram Inner Core Bead Formulation Range
[0107]
[0108] * -Compritol 888 is tribehenin and is available from Gattefosse Corp. of Paramus, NJ.
[0109] ** -Avicel PH 101 is microcrystalline cellulose and is available from FMC Corporation of Philadelphia, PA.
[0110] *** -PVP K-30 is a polyvinylpyrrolidone with a K value of about 30.
[0111] Table 2: Formulation Range of Escitalopram Modified Release Coatings
[0112]
[0113] **** -Surelease is an aqueous ethyl cellulose dispersion and is available from Colorcon, Inc. of West Point, PA.
[0114] Escitalopram inner core beads (200 mg / g) with the formulation of Table 3 were prod...
Embodiment 2
[0124] Pulse escitalopram capsule dosage form
[0125] The escitalopram cores and modified release beads described in Example 1 can be filled into capsules to give a pulsatile release profile. For example, a predetermined weight of beads can be filled into capsules using a capsule filling machine (MG-2, MG America, Fairfield, NJ). For the 4 mg strength pulsed escitalopram capsules, the number of beads per capsule is shown in Table 6.
[0126] Table 6: Formulation of pulsed escitalopram capsules
[0127] curve
[0128] Capsules containing different numbers of beads of a given strength will produce different dissolution profiles. Also, strengths corresponding to different doses can be produced by using more beads, eg 5, 8, 10, 15, 16, 20 and 40 mg in total fill weight.
Embodiment 3
[0130] Bupropion core and modified release beads
[0131] Tables 7 and 8 show the formulation ingredients and weight percentage ranges used to make the bupropion core and modified release beads, respectively. Each modified-release bead included bupropion core beads coated with a modified-release coating.
[0132] Table 7: Bupropion Core Bead Recipe Range
[0133]
[0134] Table 8: Formulation Ranges for Bupropion Modified Release Coatings
[0135]
[0136] Bupropion core beads (600 mg / g) with the formulation of Table 9 were produced.
[0137] Table 9: Bupropion core beads (600mg / g)
[0138]
[0139] The beads can be prepared by mixing ingredients 1-5 from Table 9 in a high shear granulator (Disona, Fluid Air, Chicago, IL). The pelletized material was extruded with an extruder (Disona, Fluid Air, Chicago, IL) and then spheronized into beads using a spheronizer (Disona, Fluid Air, Chicago, IL). The beads are optionally dried at 50°C for up to 12 hours.
[0140] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com