Risperidone osmotic pump controlled release tablets and preparation method thereof
An osmotic pump controlled-release, risperidone technology, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and medical preparations with non-active ingredients, etc., can solve problems such as difficulties in industrial production and increase in production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
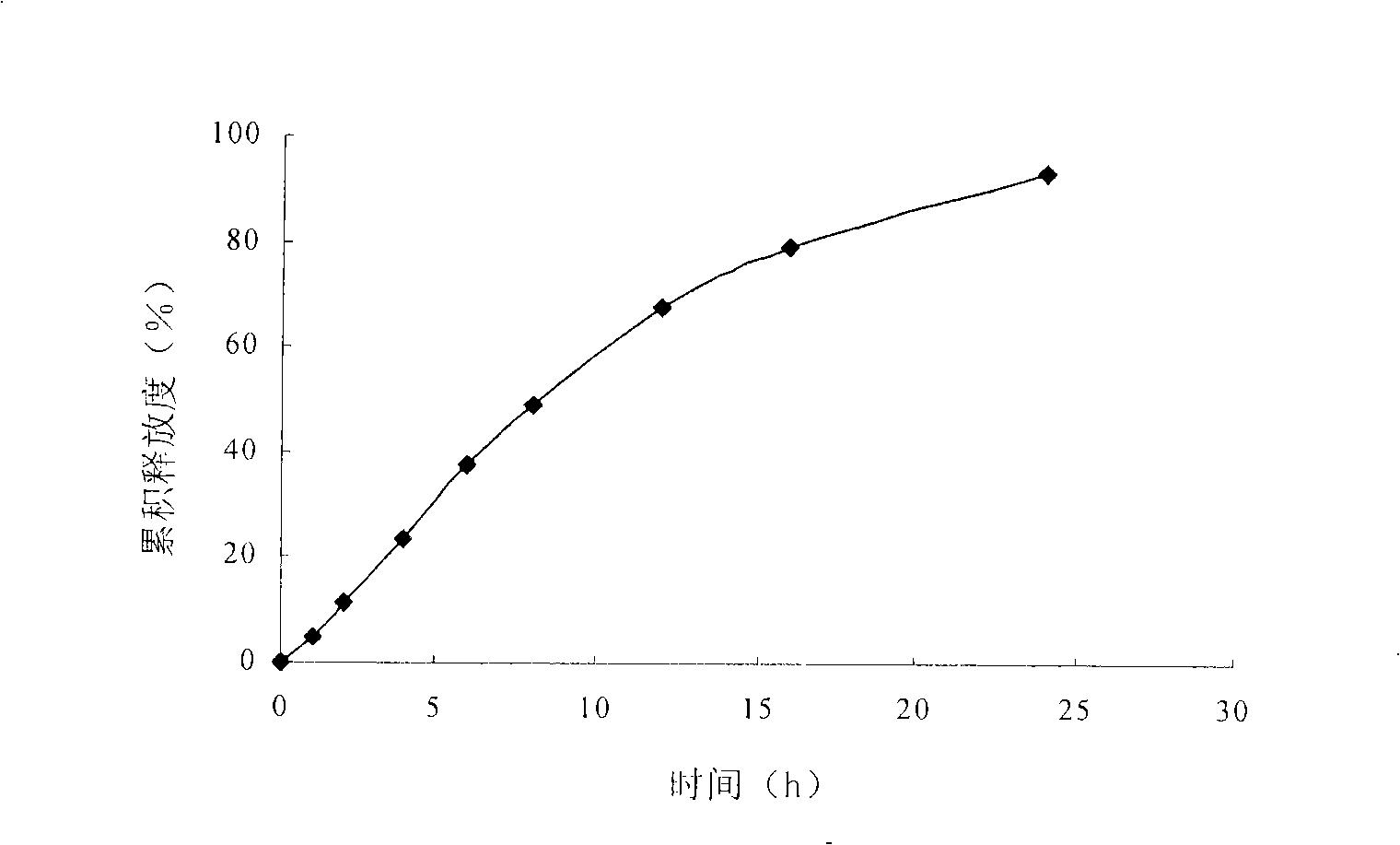
Embodiment 1
[0038] Prescription composition (1000 tablets)
[0039] Chip:
[0040] LPT 2.0g
[0041] Citric acid 2g
[0042] PVP K30 14g
[0043] HPMC 4g
[0044] Lactose 200g
[0045] Magnesium Stearate 1.5g
[0046] Coating solution:
[0047] Cellulose acetate 45mg / ml
[0048] PEG3350 5mg / ml
[0049] Acetone: water 95:5 (V / V)
[0050] Pass the risperidone and the auxiliary materials except the lubricant through a 60-mesh sieve, put them in a container and mix well. Add ethanol: water (70:30, volume ratio) to make a soft material, granulate with a 20-mesh sieve, and dry in a 45°C oven. Sieve through a 18-mesh sieve, add magnesium stearate, press into tablet cores, and place in a coating pan for coating. The coated tablet was dried in a drying oven at 50°C for 12 hours to solidify the coating film. Use a laser drilling machine to prepare a small hole with a diameter of 0.2-1.0 mm on one side of the core.
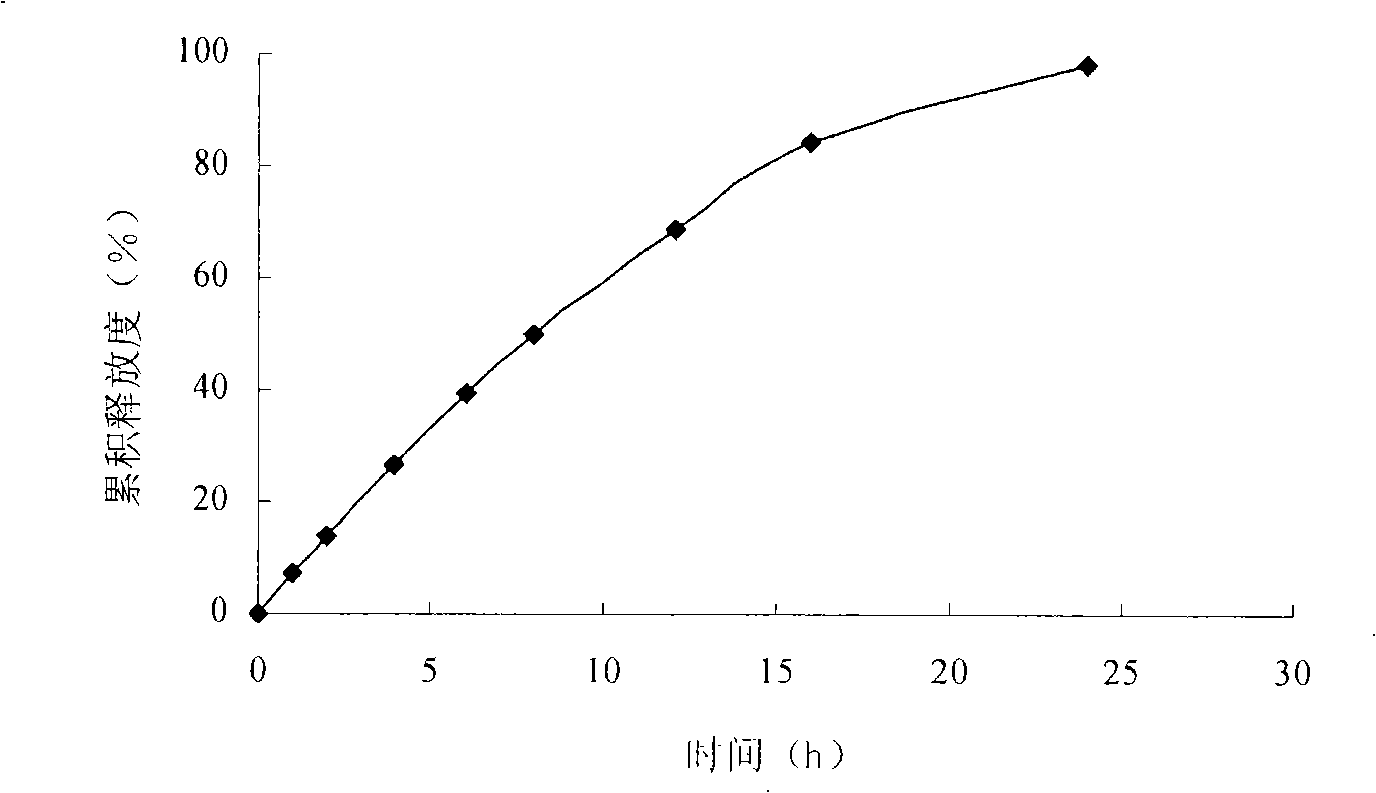
Embodiment 2
[0052] Prescription composition (1000 tablets)
[0053] Chip:
[0054] LPT 4.0g
[0055] Citric acid 4g
[0056] PVP K30 14g
[0057] HPMC 6g
[0058] Lactose 40g
[0059] Mannitol 160g
[0060] Magnesium Stearate 1.5g
[0061] Coating solution:
[0062] Cellulose acetate 45mg / ml
[0063] PEG4000 5mg / ml
[0064] Acetone: water 95:5 (V / V)
[0065] Pass the risperidone and the auxiliary materials except the lubricant through a 60-mesh sieve, put them in a container and mix well. Add ethanol: water (70:30, volume ratio) to make a soft material, granulate with a 20-mesh sieve, and dry in a 45°C oven. Sieve through a 18-mesh sieve, add magnesium stearate, press into tablet cores, and place in a coating pan for coating. The coated tablet was dried in a drying oven at 50°C for 12 hours to solidify the coating film. Use a laser drilling machine to prepare a small hole with a diameter of 0.2-1.0 mm on one side of the core.
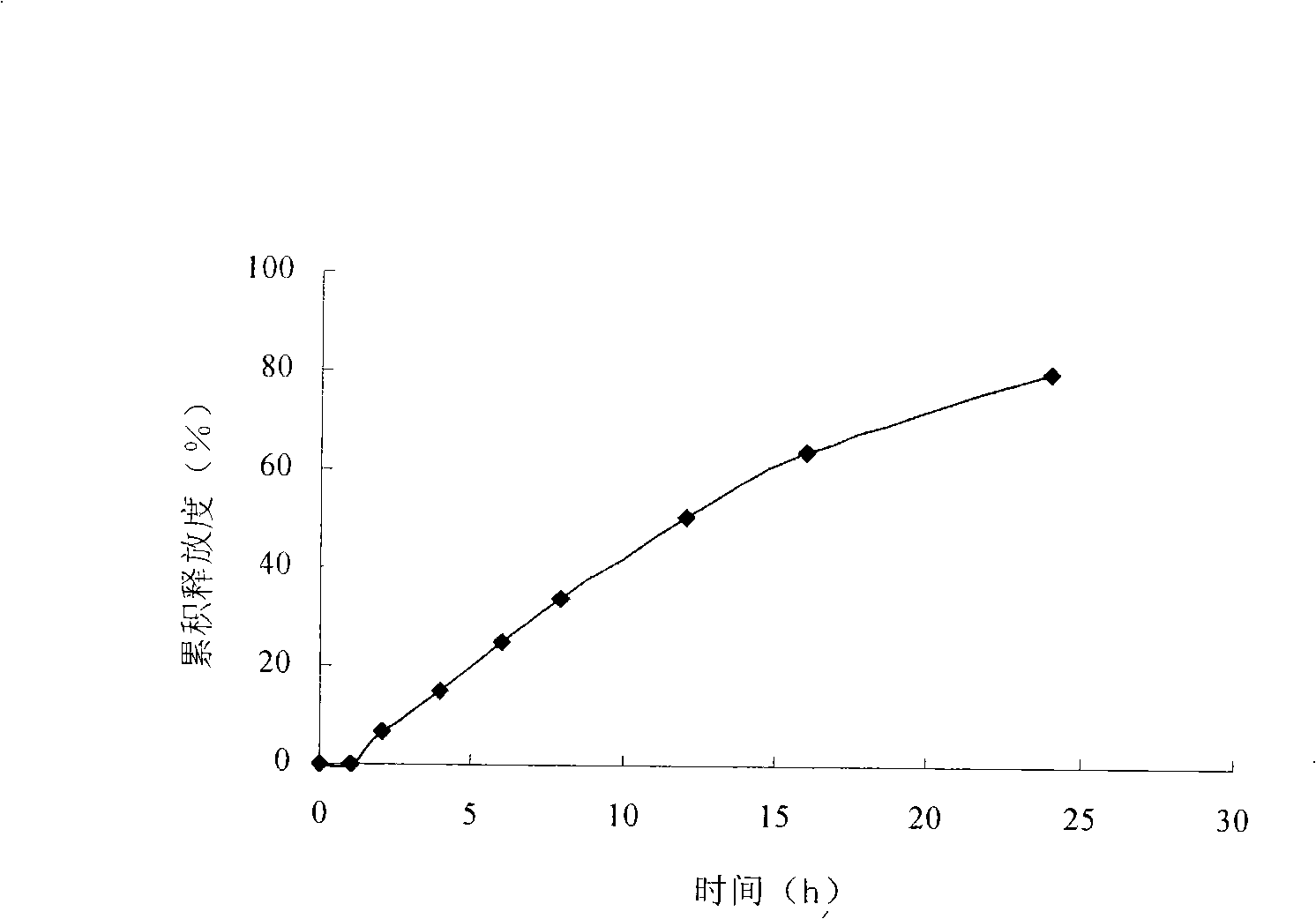
Embodiment 3
[0067] Prescription composition (based on 1000 tablets)
[0068] Drug-containing layer:
[0069] Risperidone 2.0g
[0070] Polyoxyethylene (MW20,000) 80g
[0071] NaCl 29.3g
[0072] Magnesium Stearate Appropriate amount
[0073] 10% Povidone K30 Alcohol Liquid Appropriate amount
[0074] Boost layer:
[0075] Polyoxyethylene (MW5,000,000) 95.8g
[0076] NaCl 26g
[0077] Fe 2 o 3 1.3g
[0078] Magnesium Stearate Appropriate amount
[0079] 10% Povidone K30 Alcohol Liquid Appropriate amount
[0080] Coating solution:
[0081] Cellulose acetate 48mg / ml
[0082] PEG3350 2mg / ml
[0083] Acetone: water 97:3 (V / V)
[0084] The raw and auxiliary materials of the drug-containing layer and the booster layer are respectively passed through a 60-mesh sieve according to the above ratio, and placed in containers and fully mixed. Add 10% povidone K30 alcohol solution to make soft materials respectively, granulate with 20 mesh sieve, and dry in a 45°C dry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap