Quality control method and uses of antirheumatic medicament
A detection method and anti-rheumatic technology, applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of inability to judge the quality of drugs scientifically and accurately, and achieve simple, reproducible, and exclusive methods strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1 application method provided by the present invention to the detection example of Xiaoluotong tablet
[0098] 1. The identification and determination steps are as follows:
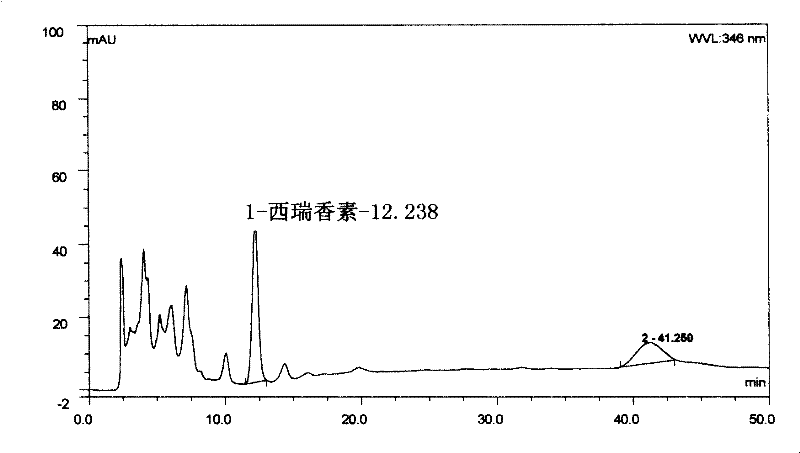
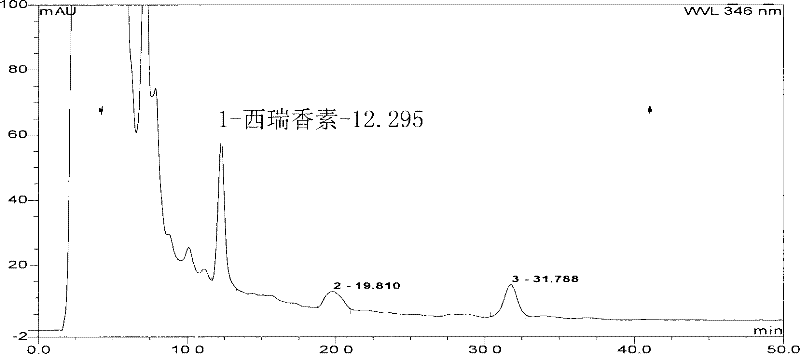
[0099] Preparation of the test solution: cancel 6 tablets of Luotong tablets, grind finely, take about 0.1-0.6g of the sample, add 5-15ml of ethanol to dissolve, extract by ultrasonic (power 100W, frequency 25kHz) for 30min, filter, and concentrate the filtrate to 1ml , as the test solution.
[0100] Preparation of the reference medicinal material solution: take 0.5 g of the reference medicinal material Daphne genkwa Tiao, and prepare the reference medicinal material solution in the same way.
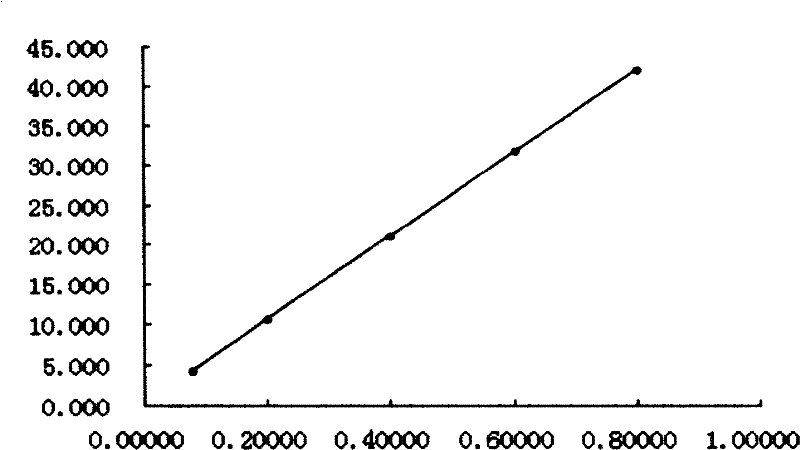
[0101] Determination by thin-layer chromatography: according to the thin-layer chromatography of appendix VIB of the Chinese Pharmacopoeia 2005 edition, draw 5 μl of each of the above two solutions, and spot them on the same silica gel G thin-layer board with sodium carboxymethylcellulose as a bi...
Embodiment 2
[0111] Embodiment 2 Application of the method provided by the present invention to the detection example of Xiaoluotong capsules
[0112] During differential determination, 3 capsules were sampled; all the other were the same as in Example 1;
[0113] When checking and measuring, it should comply with the relevant regulations under the capsules of the Chinese Pharmacopoeia;
[0114] When the extract is measured, 10 capsules are sampled; the alcohol-soluble extract content of the capsule must not be less than 18.0%; the rest are the same as in Example 1;
[0115] When determining the content, the capsule is sampled at about 0.5-1g, and each gram of the capsule contains Daphne genkwatiao and siparinin (C 19 h 12 o 7 ) meter, must not be less than 0.50mg; All the other are with embodiment 1.
Embodiment 3
[0116] Embodiment 3 Application of the method provided by the present invention to the detection example of Xiaoluotong Granules
[0117] During identification and determination, 1 to 2 bags of granules are sampled; all the other are the same as in Example 1;
[0118] When checking and measuring, it should comply with the relevant regulations under the Chinese Pharmacopoeia granules;
[0119] When the extract was measured, 10 bags of granule were sampled; the alcohol-soluble extract content of the granule should not be less than 4.0%; the rest were the same as in Example 1;
[0120] During content determination, about 2-6g of the granules are sampled, and each gram of the granules contains genkwatiao and siparinin (C 19 h 12 o 7 ) meter, must not be less than 0.10mg; All the other are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com