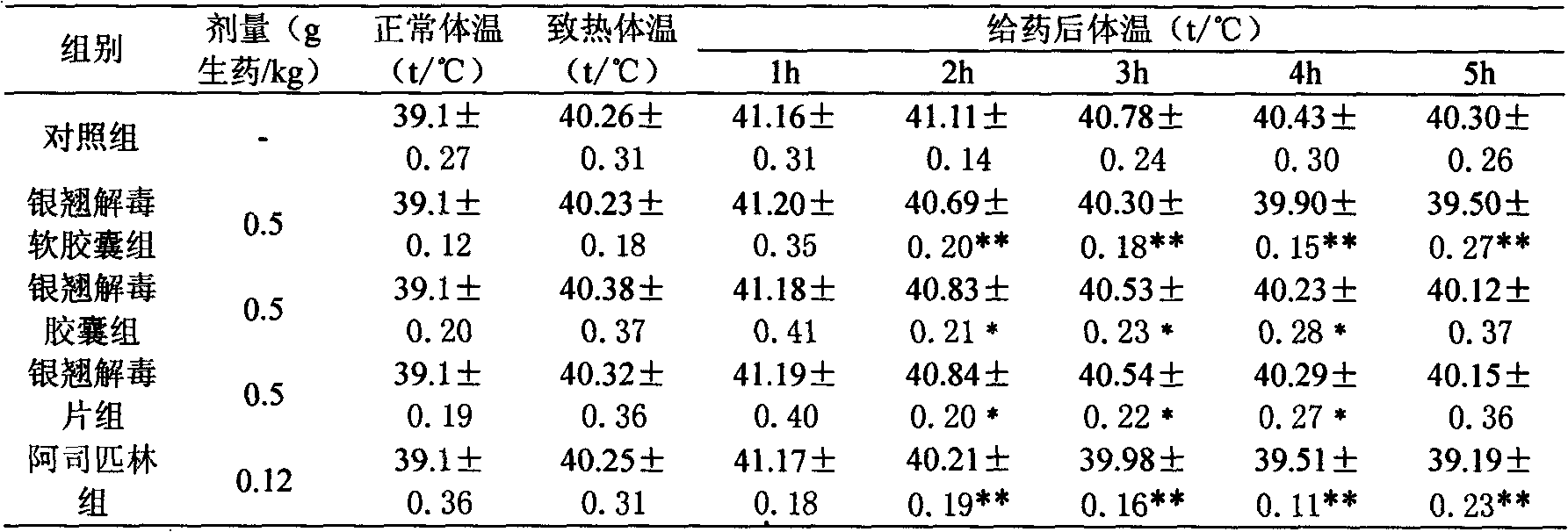
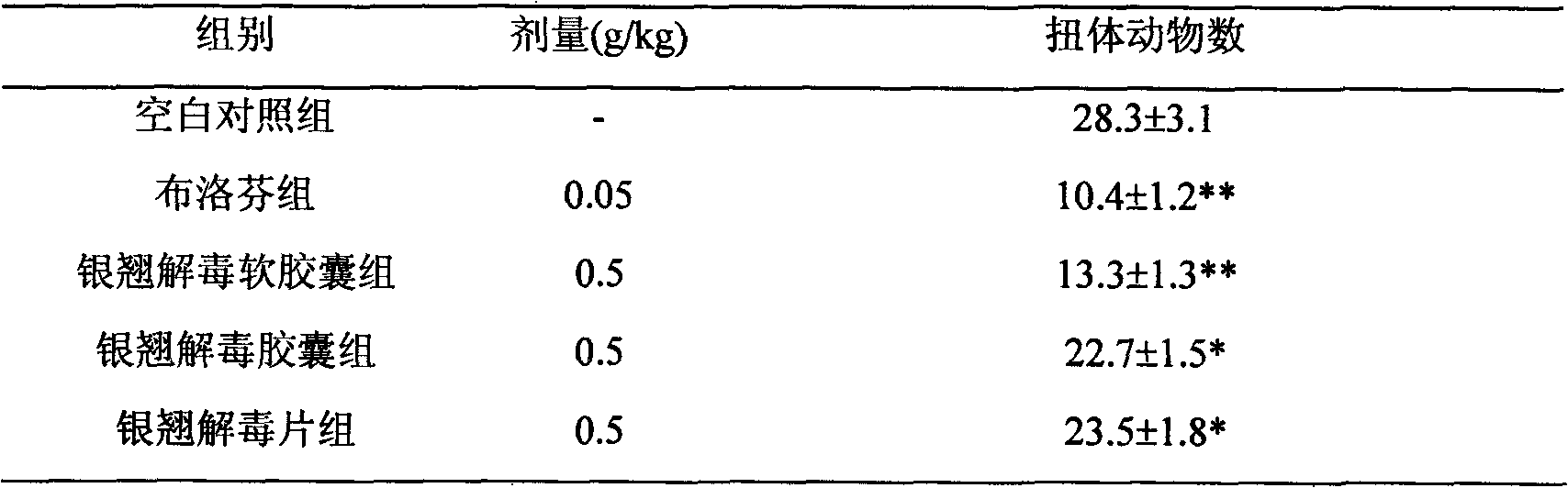
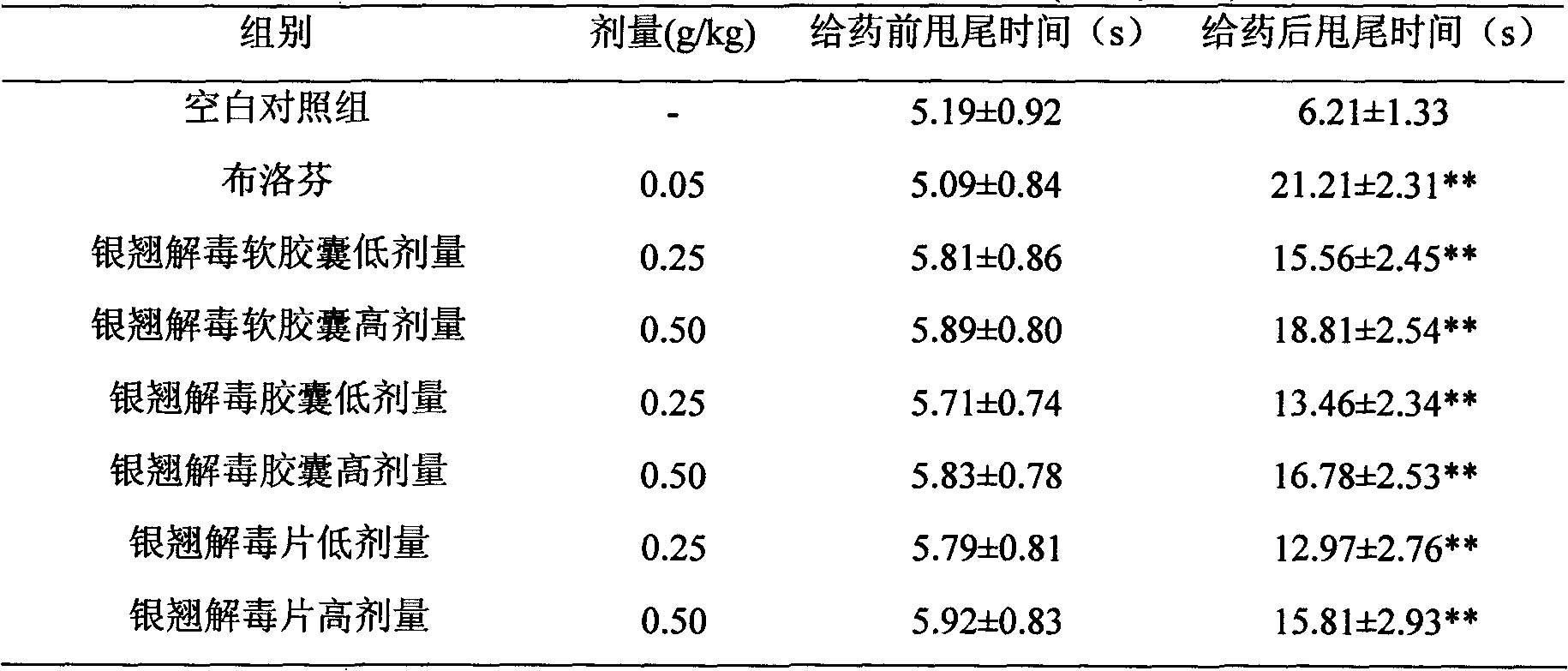
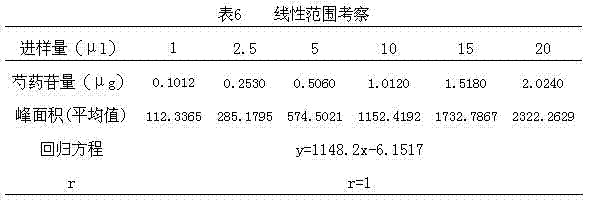
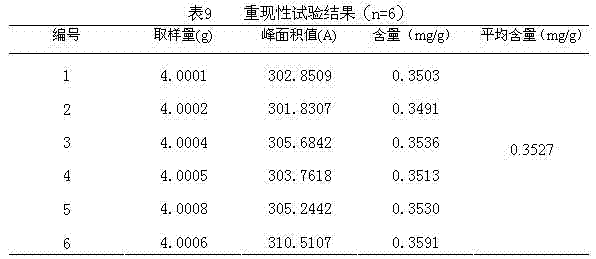
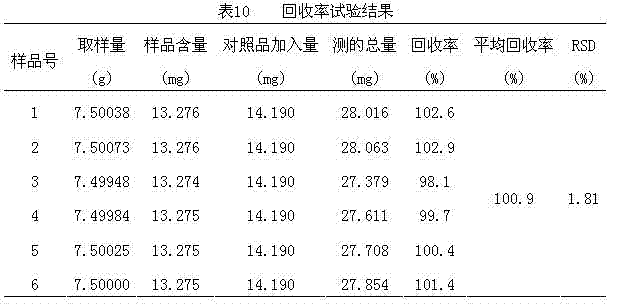
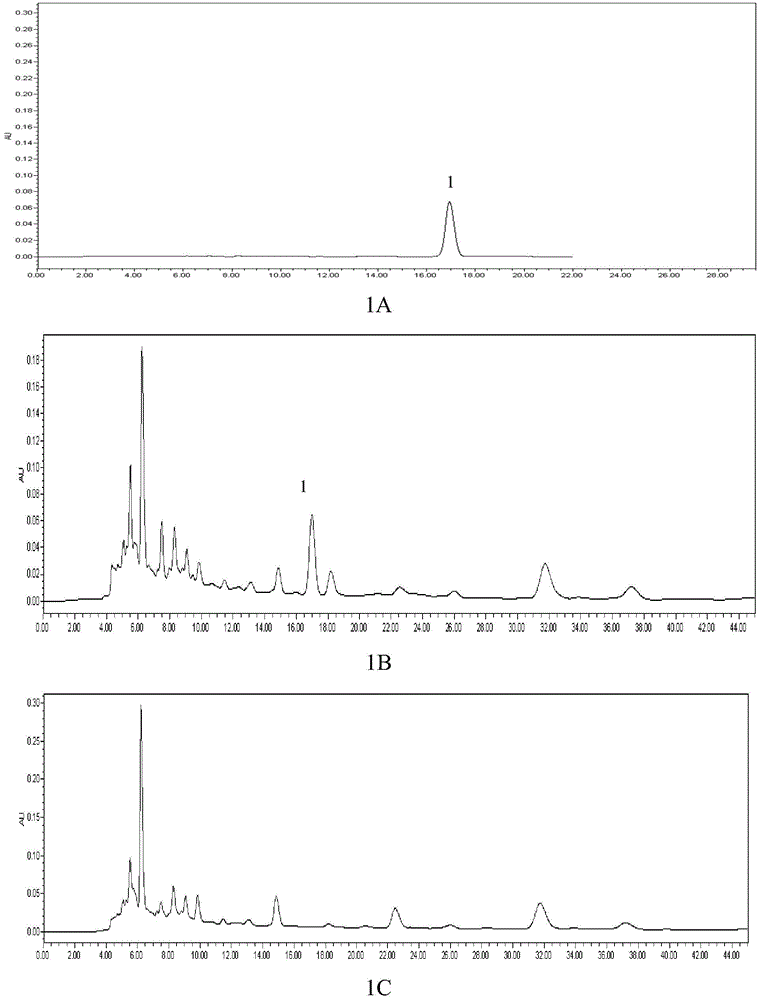
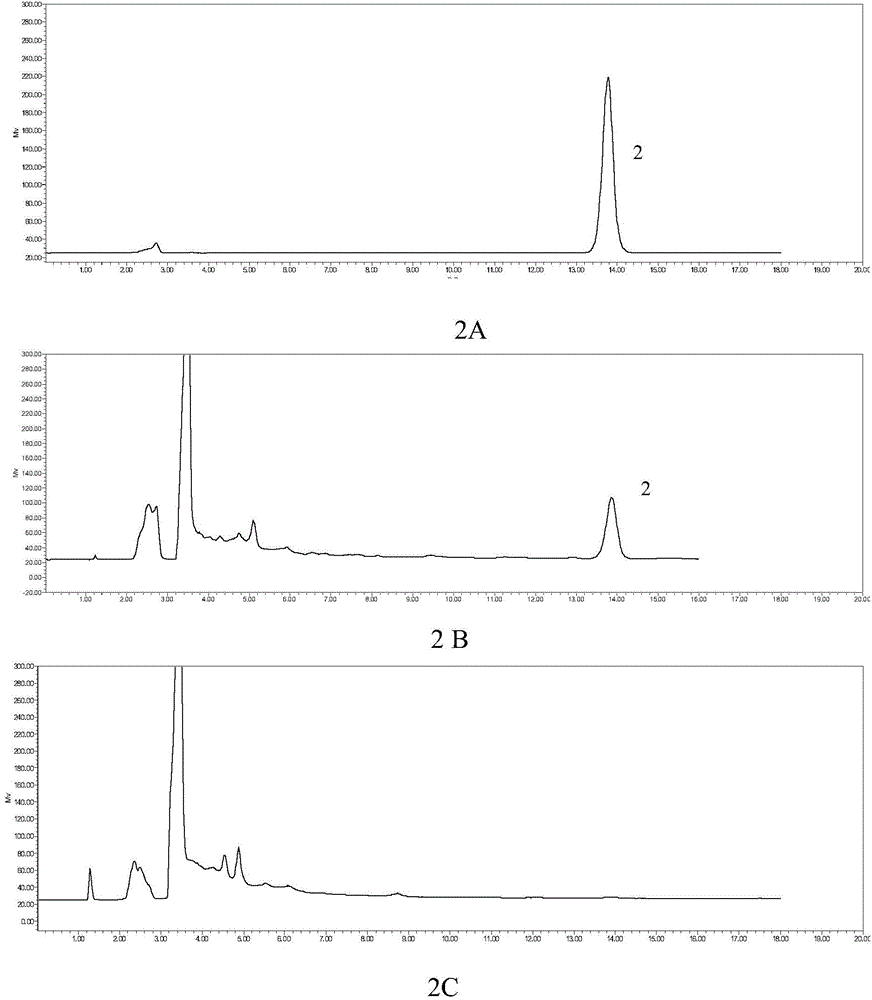
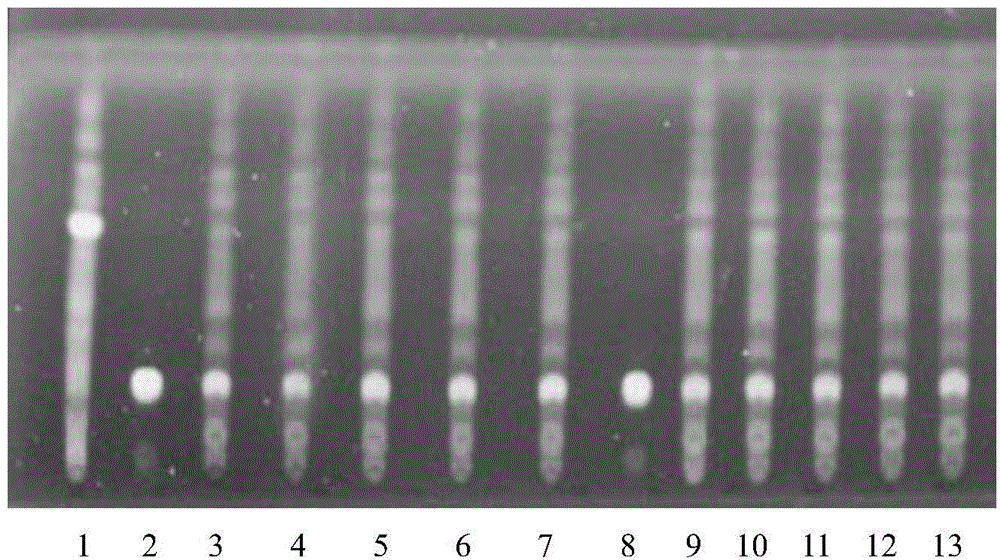
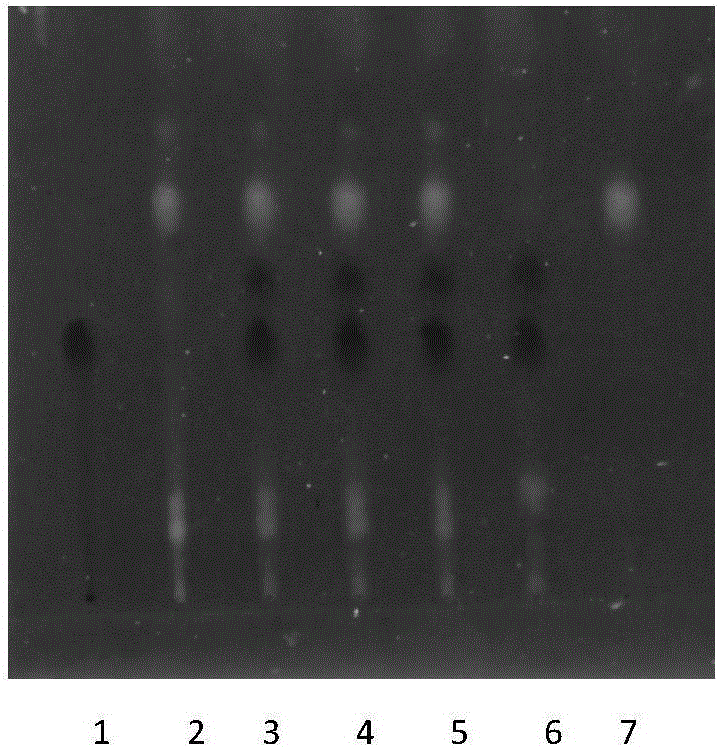
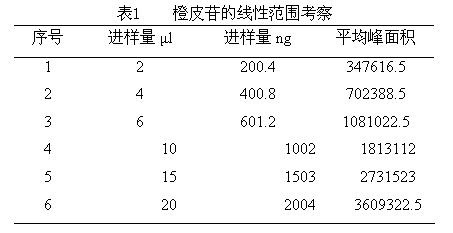
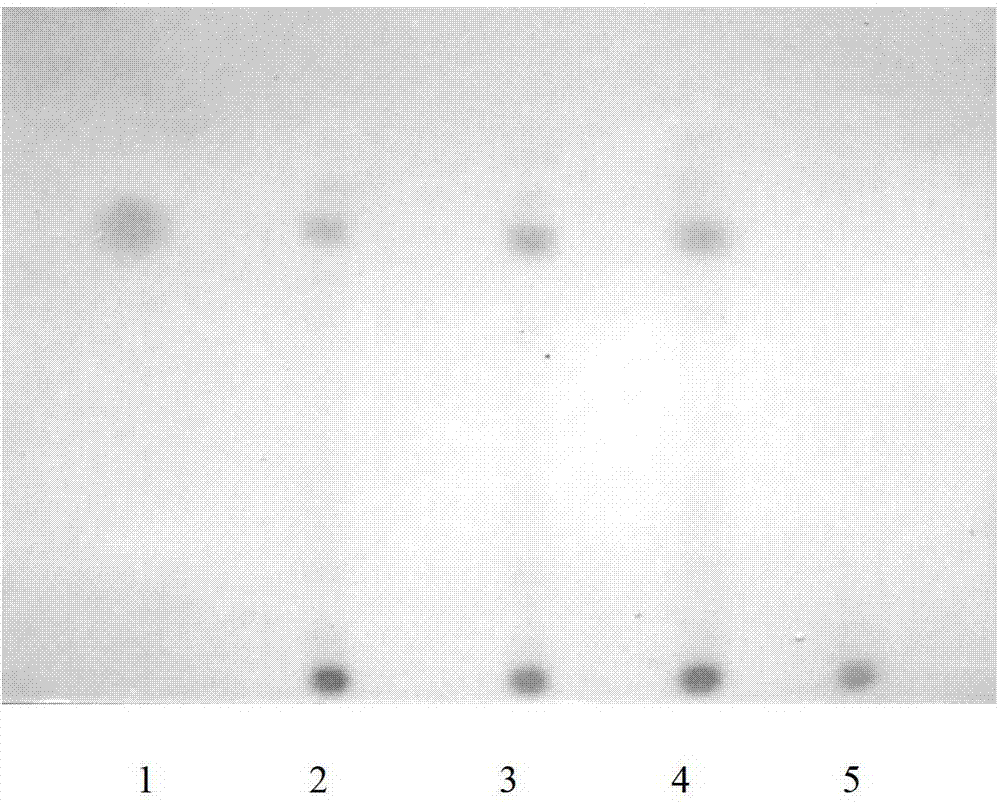
Patents
Literature
33results about How to "Negative without interference" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lonicerae and Forsythiae detoxication soft capsule medicine and preparation method and quality detection method thereof
ActiveCN101991785AQuick effectImprove bioavailabilityNervous disorderAntipyreticMedicineGLYCYRRHIZA EXTRACT
The invention relates to a Lonicerae and Forsythiae detoxication soft capsule medicine and a preparation method and a quality detection method thereof, belonging to the field of traditional Chinese medicines. The Lonicerae and Forsythiae detoxication soft capsule comprises the following raw materials in parts by weight: 5 parts of Lonicerae, 5 parts of Forsythiae, 3 parts of mint, 2 parts of Schizonepeta, 2.5 parts of fermented soya beans, 3 parts of burdock (fried), 3 parts of Platycodon grandiflorum, 2 parts of lophatherum gracile and 2.5 parts of liquorice. The invention provides the preparation method of the medicine and also provides the quality detection method, wherein the quality detection method comprises five identification methods and a method for carrying out content determination by adopting a high performance liquid chromatography, and the methods can effectively control the quality of the Lonicerae and Forsythiae detoxication soft capsule.
Owner:JIANGSU KANION PHARMA CO LTD +1
Quality control method of astragalus-leech capsules capable of regulating collaterals
InactiveCN103197027AControl drug qualityThe identification method is mature and feasibleComponent separationAstragalosideGround beetle
The invention discloses a quality control method of astragalus-leech capsules capable of regulating collaterals. The quality control method comprises the steps of: respectively differentiating leech, red peony root, astragalus root, ginseng, chuanxiong rhizome, red sage root, ground beetle, cinnamon, borneol and polygonum multiflorum in medicaments by using a thin-layer chromatography; determining the content of astragaloside in the medicaments by using a thin-layer scanning method; and determining the content of tanshinol and paeoniflorin in the medicaments by using a liquid-phase chromatography. According to the quality control method disclosed by the invention, the medicinal material differentiation method is mature, feasible, negative and non-interfering and is strong in specificity, and the content determination method is easy to operate, high in precision and good in reproducibility, so that the quality control method disclosed by the invention can be used for controlling the quality of the medicaments accurately and stably, thereby being adaptable to the industrial stable production of the medicaments.
Owner:山西振东五和堂制药有限公司
Quality control method for rhizoma gastrodiae capsule
ActiveCN104306745AToxicEasy to emulsifySenses disorderNervous disorderMedicineThin layer chromatographic
The invention relates to a quality control method for a rhizoma gastrodiae capsule and belongs to the technical field of biomedicine. The quality control method comprises the steps of raw material control, preparation method control, an identification method and a content measurement method. Modern drug production management is integrated in quality control. The quality of drugs and solvents used in production is examined from the source. Product quality control in the production process is established, perfect product quality standards are established, the thin-layer chromatographic detection ratio is higher than national requirements, the gastrodin content is greatly increased, the increase degree reaches 28.3%, and the quality and the therapeutic effect of the drug product are ensured.
Owner:YUNNAN YONGZITANG PHARMA
Quality control method of granules capable of strengthening and consolidating body resistance
InactiveCN103197026AStrong specificityEasy to operateComponent separationThin-layer chromatographyQuality control
The invention discloses a quality control method of granules capable of strengthening and consolidating body resistance. The quality control method comprises the steps of: respectively differentiating scutellaria root, ligustrum lucidum, polygonum multiflorum, epimedium and madder in medicaments by using a thin-layer chromatography; and determining the content of baicalin, icariin and emodin in the medicaments by using a liquid-phase chromatography. According to the quality control method disclosed by the invention, the medicinal material differentiation method is mature, feasible, negative and non-interfering and is strong in specificity, and the content determination method is easy to operate, high in precision and good in reproducibility, so that the quality control method disclosed by the invention can be used for controlling the quality of the medicaments accurately and stably, thereby being adaptable to the industrial stable production of the medicaments.
Owner:山西振东五和堂制药有限公司
Detection method of Jiuwei Zhuhuang preparation
ActiveCN102590212AEfficient detectionStrong specificityComponent separationMaterial analysis by optical meansThin-layer chromatographyChemistry
The invention relates to a detection method of a Jiuwei Zhuhuang preparation, comprising one or several kinds of the following identification and / or detection methods: identifying morphological characteristics of liquorice and safflower in the preparation by a microscope; qualitatively identifying the safflower, the liquorice and artificial cow-bezoar in the preparation by thin-layer chromatography; detecting the content of aconitine contained in bonga aconite in the preparation; and measuring the content of the hydroxyl carthamin yellow A contained in the safflower in the preparation by high performance liquid chromatography. The Jiuwei Zhuhuang preparation is effectively detected by the above detection method.
Owner:TIBET QIZHENG TIBETAN MEDICINE
Detection method of pharmaceutical composition Xianyu for treating epileptoid convulsions, infantile convulsions and facial spasms
ActiveCN104569166AProtection against convulsionsProtection Sedation HypnosisNervous disorderComponent separationAstragalosideConvulsion
The invention provides a detection method of pharmaceutical composition Xianyu for treating epileptoid convulsions, infantile convulsions and facial spasms. The detection method comprises steps as follows: detecting the content of gastrodin in the pharmaceutical composition with a liquid chromatography; detecting the content of astragaloside in the pharmaceutical composition with an HPLC (high performance liquid chromatography); detecting salvianic acid A sodium in the pharmaceutical composition with a TLC (thin-layer chromatography). With the adoption of the detection method, the quality of the pharmaceutical composition can be controlled more accurately and can be stable, controllable, efficient and safe, defects in the prior art can be overcome, and the powerful guarantee can be provided for well satisfaction of medical requirements.
Owner:XIAN CHIHO PHARMA
Detection method of traditional Chinese medicine preparation for preventing infectious bronchitis
The invention provides a detection method of a traditional Chinese medicine preparation for preventing infectious bronchitis. The traditional Chinese medicine preparation is prepared from roots of Baikal skullcap, fructus forsythia, cape jasmine fruits, honeysuckle, pogostemon cablin, Chinese thorowax roots and Thunberg fritillary bulb. The quality detection method includes identification and content determination projects. The thin-layer chromatography identification method for seven traditional Chinese medicines is clear in spot, good in repeatability, negative and free from interference, and can serve as a traditional Chinese medicine composition quality control method. A built baicalein content determination method is high in precision and accurate in measuring result, and product quality can be effectively controlled, so that clinical curative effects are ensured.
Owner:中悦兴农(北京)科技有限公司
Quality control method of infant spleen tonifying medicament
ActiveCN103163272AStrong specificityEasy to operateComponent separationPreparing sample for investigationThin-layer chromatographyDrug
The invention discloses a quality control method of an infant spleen tonifying medicament. According to the method, hawthorn and licorice in the medicament are identified by using layer chromatography respectively, and the hesperidin content of the medicament is measured by using liquid chromatography. In the established quality control method, the medicinal material identification method is mature, feasible, high in specificity, negative and non-interfering; the content measurement method is easy to operate, high in precision and high in reproducibility; and by adopting the quality control method, the quality of the medicament can be accurately and stably controlled to adapt to industrialized stable production of the medicament.
Owner:山西振东五和堂制药有限公司
Quality detection method of capsule for reducing blood fat and activating collaterals
InactiveCN102133377AFor the purpose of qualitative controlTo achieve the purpose of qualitative controlMetabolism disorderComponent separationCassiaThin layer chromatographic
The invention discloses a quality detection method of a capsule for reducing blood fat and activating collaterals. The capsule for reducing blood fat and activating collaterals is prepared from 100g of cassia seed, 1500g of curcuma, 2000g of rhizoma alismatis, 500g of notoginseng and 2000g of bermudagrass herb. The quality detection method of the capsule for reducing blood fat and activating collaterals additionally provides thin-layer chromatography identification of the rhizoma alismatis on the basis of the primary standard, and the rhizoma alismatis is one of main components of the capsule. After the thin-layer chromatography identification of the rhizoma alismatis is additionally provided on the basis of the primary standard, the purpose of qualitatively controlling the rhizoma alismatis in the preparation is achieved, the quality detection standard of the capsule for reducing blood fat and activating collaterals is perfected, and simultaneously, a scientific basis for judging the truth of products and controlling the quality of products is provided. In the method, a sample test is simple and convenient to operate; and proven by negative interference and other continuous verification tests, the method has the advantages of negative non-interference, good reproducibility and strong specificity. The invention perfects the quality detection standard and improves the control capability of the product quality.
Owner:唐秋海
Detection method of yupingfeng oral liquid
ActiveCN103308645AEasy to judgeShorten identification timeComponent separationPretreatment methodMedicine
The invention relates to a detection method of a yupingfeng oral liquid. A thin layer chromatography is adopted, the same sample pretreatment method and the same chromatographic condition are used, and astragali radix and saposhnidoviae radix in a yupingfeng oral liquid prescription are simultaneously identified on the same thin layer plate. The method of identifying the astragali radix and the saposhnidoviae radix by the thin layer chromatography comprises the steps of (1) test solution preparation, (2) reference solution preparation, and (3) thin layer chromatography conditions and result. According to the detection method, the quality standard of the yupingfeng oral liquid is improved and perfected, and the identification of the saposhnidoviae radix is revised from a high performance liquid chromatography to a thin layer identification method, so that the identification time is greatly shortened, and the detection cost is lowered. Compared with a method in the prior art, the detection method is simpler, more convenient, rapider and more economical; the quality is easier to control; and the detection method is more suitable for industrial production.
Owner:BAODING JIZHONG PHARMA
Thin-layer chromatography method for simultaneously identifying four single medicinal materials from Lonicera and Forsythia powder
ActiveCN113484456AShorten identification timeReduce processing timeComponent separationMedicinal herbsThin layer chromatographic
The invention discloses a thin-layer chromatography method for simultaneously identifying four single medicinal materials from Lonicera and Forsythia powder, which comprises the following steps of: weighing Lonicera and Forsythia powder, adding methanol for extracting, filtering, evaporating filtrate to dryness in a water bath, adding water to dissolve residues, extracting by using a water-saturated n-butyl alcohol solution, adding water to wash a separated n-butyl alcohol layer, discarding water liquid, adding petroleum ether, layering, collecting an upper-layer solution, adding a methanol solution into the upper-layer solution, washing, collecting an upper-layer solution, drying by distillation, and dissolving residues with methanol to a constant volume of 0.5-2mL. According to the thin-layer chromatography identification method, as long as one test solution is prepared, the four medicinal materials including arctii fructus, liquorice, semen sojae praeparatum and schizonepetae spica can be simultaneously identified from a complex system of the traditional Chinese medicine Lonicera and Forsythia powder under two development conditions. The thin-layer identification time is shortened, the detection efficiency is improved, the consumption of reference substances and reagents is reduced, and the detection cost is greatly reduced.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
A detection method for Gastrodia Xingnao Capsules
ActiveCN104306745BToxicEasy to emulsifySenses disorderNervous disorderThin layer chromatographicTherapeutic effect
Owner:YUNNAN YONGZITANG PHARMA
Cabrite medicinal materials discrimination method
ActiveCN101210915AThe identification method is matureNegative without interferenceComponent separationPreparing sample for investigationFluorescenceSolvent
The invention relates to a method for identifying a medicinal lizard material, which comprises the following steps of: selecting a reference lizard material as the reference, respectively preparing a test sample solution and a reference material solution, dispensing on the same silica gel G thin layer plate, developing by a developing solvent including toluene or benzene and ethyl acetate with a ratio of 1:(0.8-1.3), adding ethanol sulfate solution so that the test sample and the reference material render the identical color at corresponding positions, and visually inspecting using 365 nm UV light under UV lamp to observe fluorescent spots of the same color of the test sample and the reference material at corresponding positions. The inventive identification method has high specificity and good reproducibility, and is suitable for identification of lizard materials of all resources and for identification of the presence or not of lizard material in products prepared from lizard materials of all resources.
Owner:山西华元医药生物技术有限公司
Rapid thin-layer identification method for multiple medicines in common goldenrop granule finished products
ActiveCN111830187AReduce consumptionShorten identification timeComponent separationAgainst vector-borne diseasesMedicinal herbsThin layer chromatographic
The invention discloses a rapid thin-layer identification method for multiple medicines in common goldenrop granule finished products. The rapid thin-layer identification method comprises the following steps: S1, preparing a reference medicinal material solution, a reference substance solution and a test solution; S2, carrying out thin-layer chromatography identification; and S3, carrying out methodology verification and finished product detection. According to the method, the same test solution and the same developing solvent can be used for simultaneously identifying the bupleurum chinense (monarch drug), scutellaria baicalensis (minister drug) and liquorice (assistant and guide drug) in the common goldenrop granule finished products on a thin-layer plate, so that the identification timeis shortened, the detection efficiency is improved, the consumption of reagents is reduced, and the detection cost is greatly reduced.
Owner:鉴甄检测技术(上海)有限公司
Identification method of herba patriniae in Chinese herbal compound
ActiveCN106501440AAdvantages of identification methodEasy to separateComponent separationPreparing sample for investigationMedicineThin layer
The invention discloses an identification method of herba patriniae in a Chinese herbal compound, and particularly relates to a thin-layer identification method of herba patriniae in a Chinese herbal compound. The identification method comprises the following steps of preparing a reference crude drug solution; preparing a test product solution; preparing a negative sample solution; performing sample application on the same silica gel G thin-layer plate with a capillary sample applicator; placing the thin-layer plate into a chromatographic jar, performing expansion with an expanding agent, taking out and drying the thin-layer plate in air; and inspecting a thin-layer chromatographic sheet to obtain a result for identification, wherein a main spot of the same color is developed at a position, which corresponds to a reference crude drug chromatography, on a test product chromatography, but no spot is developed on a negative sample. The identification method disclosed by the invention is simple in technology, good in separation effect and extremely high in specificity; and the accuracy of component identification of herba patriniae in the Chinese herbal compound is further improved, and the identification method has a huge popularization and application potential in the field of identification of herba patriniae-related medicines.
Owner:HEBEI KEHENG BIOTECH
Detection method of Xianyu capsule for treating epileptoid convulsion, infantile convulsion and mimic convulsion
ActiveCN103592406AQuality assuranceQuality improvementNervous disorderAnthropod material medical ingredientsConvulsionSalvia miltiorrhiza
The invention provides a detection method of a medicine for treating epileptoid convulsion, infantile convulsion and mimic convulsion. The detection method comprises a step that Salvia miltiorrhiza in the medicine by adopting thin layer chromatography, wherein thin layer chromatography conditions are characterized in that a silica gel G thin layer plate is adopted, and an A-B-C mixed solution having an A / B / C volume ratio of 70-100:5-10:1-5, preferably 80-100:6-9:1-4, and more preferably 90:8:2 is adopted as a developing solvent; the above A solution is selected from dichloromethane, benzene, toluene, trichloromethane, isopropanol and n-butanol, and preferably trichloromethane; the above B solution is selected from ethanol, ethyl acetate, acetone, glycol, methanol and dioxin, and preferably methanol; and the above C solution is selected from formic acid, acetate acid gracial, dimethyl ether, methyl formate and water, and preferably formic acid. The method allows the quality of the medicine to be effectively controlled, and to be stable, controllable, efficient and safe.
Owner:XIAN CHIHO PHARMA
Quality control method of loins-strengthening and kidney-invigorating medicine
ActiveCN103149320BAdapt to industrialized stable productionThe identification method is mature and feasibleComponent separationMedicineQuality control
Owner:山西振东五和堂制药有限公司
A detection method for cough medicine
ActiveCN103175938BAdapt to industrialized stable productionQuality control method controlComponent separationMedicineQuality control
The invention discloses a quality control method of a drug for treating cough. The quality control method comprises the following steps of: respectively indentifying borneol, immature fruits of terminalia chebula, ephedra, root barks of white mulberry and radix scutellariae in the drug by using thin layer chromatography, and measuring the content of ephedrine hydrochloride in the drug by using liquid chromatography. The quality control method established by the invention has the advantages that a crude drug indentifying method is mature and feasible, strong in specificity, negative and free of interference; a content measuring method is easy to operate, high in precision and good in repeatability; and by using the quality control method disclosed by the invention, the quality of the drug can be precisely and stably controlled so that the requirement for stable industrial production of the drug is met.
Owner:山西振东五和堂制药有限公司
Lonicerae and Forsythiae detoxication soft capsule medicine and preparation method and quality detection method thereof
ActiveCN101991785BQuick effectImprove bioavailabilityNervous disorderAntipyreticMedicineGLYCYRRHIZA EXTRACT
The invention relates to a Lonicerae and Forsythiae detoxication soft capsule medicine and a preparation method and a quality detection method thereof, belonging to the field of traditional Chinese medicines. The Lonicerae and Forsythiae detoxication soft capsule comprises the following raw materials in parts by weight: 5 parts of Lonicerae, 5 parts of Forsythiae, 3 parts of mint, 2 parts of Schizonepeta, 2.5 parts of fermented soya beans, 3 parts of burdock (fried), 3 parts of Platycodon grandiflorum, 2 parts of lophatherum gracile and 2.5 parts of liquorice. The invention provides the preparation method of the medicine and also provides the quality detection method, wherein the quality detection method comprises five identification methods and a method for carrying out content determination by adopting a high performance liquid chromatography, and the methods can effectively control the quality of the Lonicerae and Forsythiae detoxication soft capsule.
Owner:JIANGSU KANION PHARMA CO LTD +1
Quality control method of infant spleen tonifying medicament
ActiveCN103163272BAdapt to industrialized stable productionControl drug qualityComponent separationPreparing sample for investigationMedicineSpleen
The invention discloses a quality control method of an infant spleen tonifying medicament. According to the method, hawthorn and licorice in the medicament are identified by using layer chromatography respectively, and the hesperidin content of the medicament is measured by using liquid chromatography. In the established quality control method, the medicinal material identification method is mature, feasible, high in specificity, negative and non-interfering; the content measurement method is easy to operate, high in precision and high in reproducibility; and by adopting the quality control method, the quality of the medicament can be accurately and stably controlled to adapt to industrialized stable production of the medicament.
Owner:山西振东五和堂制药有限公司
Detection method of Jiuwei Zhuhuang preparation
ActiveCN102590212BEfficient detectionStrong specificityComponent separationMaterial analysis by optical meansBiotechnologyAconitum carmichaeli
The invention relates to a detection method of a Jiuwei Zhuhuang preparation, comprising one or several kinds of the following identification and / or detection methods: identifying morphological characteristics of liquorice and safflower in the preparation by a microscope; qualitatively identifying the safflower, the liquorice and artificial cow-bezoar in the preparation by thin-layer chromatography; detecting the content of aconitine contained in bonga aconite in the preparation; and measuring the content of the hydroxyl carthamin yellow A contained in the safflower in the preparation by high performance liquid chromatography. The Jiuwei Zhuhuang preparation is effectively detected by the above detection method.
Owner:TIBET QIZHENG TIBETAN MEDICINE
Quality control method and uses of antirheumatic medicament
ActiveCN101366875BEffective quality controlThe method is simple and fastComponent separationAntipyreticAlcoholMedicine
Owner:RONGCHANG PHARM ZIBO CO LTD
Identification method of Patrinia in traditional Chinese medicine compound
ActiveCN106501440BEasy to separateNegative without interferenceComponent separationPreparing sample for investigationMedicinal herbsMedicine
The invention discloses an identification method of herba patriniae in a Chinese herbal compound, and particularly relates to a thin-layer identification method of herba patriniae in a Chinese herbal compound. The identification method comprises the following steps of preparing a reference crude drug solution; preparing a test product solution; preparing a negative sample solution; performing sample application on the same silica gel G thin-layer plate with a capillary sample applicator; placing the thin-layer plate into a chromatographic jar, performing expansion with an expanding agent, taking out and drying the thin-layer plate in air; and inspecting a thin-layer chromatographic sheet to obtain a result for identification, wherein a main spot of the same color is developed at a position, which corresponds to a reference crude drug chromatography, on a test product chromatography, but no spot is developed on a negative sample. The identification method disclosed by the invention is simple in technology, good in separation effect and extremely high in specificity; and the accuracy of component identification of herba patriniae in the Chinese herbal compound is further improved, and the identification method has a huge popularization and application potential in the field of identification of herba patriniae-related medicines.
Owner:HEBEI KEHENG BIOTECH
Detection method of yupingfeng oral liquid
ActiveCN103308645BEasy to judgeShorten identification timeComponent separationPretreatment methodMedicine
The invention relates to a detection method of a yupingfeng oral liquid. A thin layer chromatography is adopted, the same sample pretreatment method and the same chromatographic condition are used, and astragali radix and saposhnidoviae radix in a yupingfeng oral liquid prescription are simultaneously identified on the same thin layer plate. The method of identifying the astragali radix and the saposhnidoviae radix by the thin layer chromatography comprises the steps of (1) test solution preparation, (2) reference solution preparation, and (3) thin layer chromatography conditions and result. According to the detection method, the quality standard of the yupingfeng oral liquid is improved and perfected, and the identification of the saposhnidoviae radix is revised from a high performance liquid chromatography to a thin layer identification method, so that the identification time is greatly shortened, and the detection cost is lowered. Compared with a method in the prior art, the detection method is simpler, more convenient, rapider and more economical; the quality is easier to control; and the detection method is more suitable for industrial production.
Owner:BAODING JIZHONG PHARMA
A kind of assay method of Qianlieping capsule
ActiveCN110763782BEffective quality controlGood precisionComponent separationFluid phaseCurative effect
Owner:XIAN CHIHO PHARMA
A detection method of Qianlieping Capsules for treating acute and chronic prostatitis
ActiveCN103592405BStrong discriminative specificityHigh sensitivityComponent separationN-ButanolEthyl acetate
The invention provides a detection method of a Qianlieping capsule for treating acute and chronic prostatitis. The detection method comprises a step that safflower in the above medicine is detected by adopting thin layer chromatography, wherein thin layer chromatography conditions are characterized in that a silica gel GF254 thin layer plate is adopted, and an A-B-C mixed solution having an A / B / C volume ratio of 5-12:1-5:0.1-0.7, preferably 7-11:1-3:0.1-0.5, and more preferably 9:2:0.5 is adopted as a developing solvent; the above A solution is selected from benzene, toluene, cyclohexane, trichloromethane, dichloromethane and n-butanol , and preferably cyclohexane; the above B solution is selected from acetone, ethyl acetate, butyl acetate, methanol and glycol, and preferably ethyl acetate; and the above C solution is selected from water, methyl formate, dimethyl ether, acetate acid gracial and formic acid, and preferably acetate acid gracial. The method allows the quality of the medicine to be effectively controlled, and to be stable, controllable, efficient and safe.
Owner:XIAN CHIHO PHARMA
A method for detecting children's Huaji granules
ActiveCN109596768BIdentification spots are clearNegative without interferenceChemical analysis using titrationComponent separationOrganic acidArecoline
Disclosed is a quality control method for infantile accumulation-eliminating granules. The method comprises: using thin-layer chromatography to perform qualitative identification on parched Crataegi fructus and Pharbitidis semen in infantile accumulation-eliminating granules; using high-performance liquid chromatography to determine the content of an activation component, arecoline, in the infantile accumulation-eliminating granules to carry out quantitative control; and using a potentiometric titration method to determine the content of organic acids in the infantile accumulation-eliminating granules. The method of the present invention is simple, has a good degree of separation, strong specificity and good reproducibility, thereby effectively guaranteeing the quality and curative effect of the infantile accumulation-eliminating granules, and having very strong practicality.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
A detection method of Xianyu Capsules for the treatment of epileptic convulsions, infantile convulsions, and hemifacial spasms
ActiveCN103592406BHigh sensitivityGood repeatabilityNervous disorderAnthropod material medical ingredientsSalvia miltiorrhizaConvulsion
Owner:XIAN CHIHO PHARMA
A method for detecting epilepsy recovery of a pharmaceutical composition for treating epileptic convulsions, infantile convulsions, and hemifacial spasm
ActiveCN104569166BProtection against convulsionsProtection Sedation HypnosisNervous disorderComponent separationConvulsionAstragaloside
The invention provides a detection method of pharmaceutical composition Xianyu for treating epileptoid convulsions, infantile convulsions and facial spasms. The detection method comprises steps as follows: detecting the content of gastrodin in the pharmaceutical composition with a liquid chromatography; detecting the content of astragaloside in the pharmaceutical composition with an HPLC (high performance liquid chromatography); detecting salvianic acid A sodium in the pharmaceutical composition with a TLC (thin-layer chromatography). With the adoption of the detection method, the quality of the pharmaceutical composition can be controlled more accurately and can be stable, controllable, efficient and safe, defects in the prior art can be overcome, and the powerful guarantee can be provided for well satisfaction of medical requirements.
Owner:XIAN CHIHO PHARMA
Quality detection method of Chinese angelica oral liquid for benefiting blood
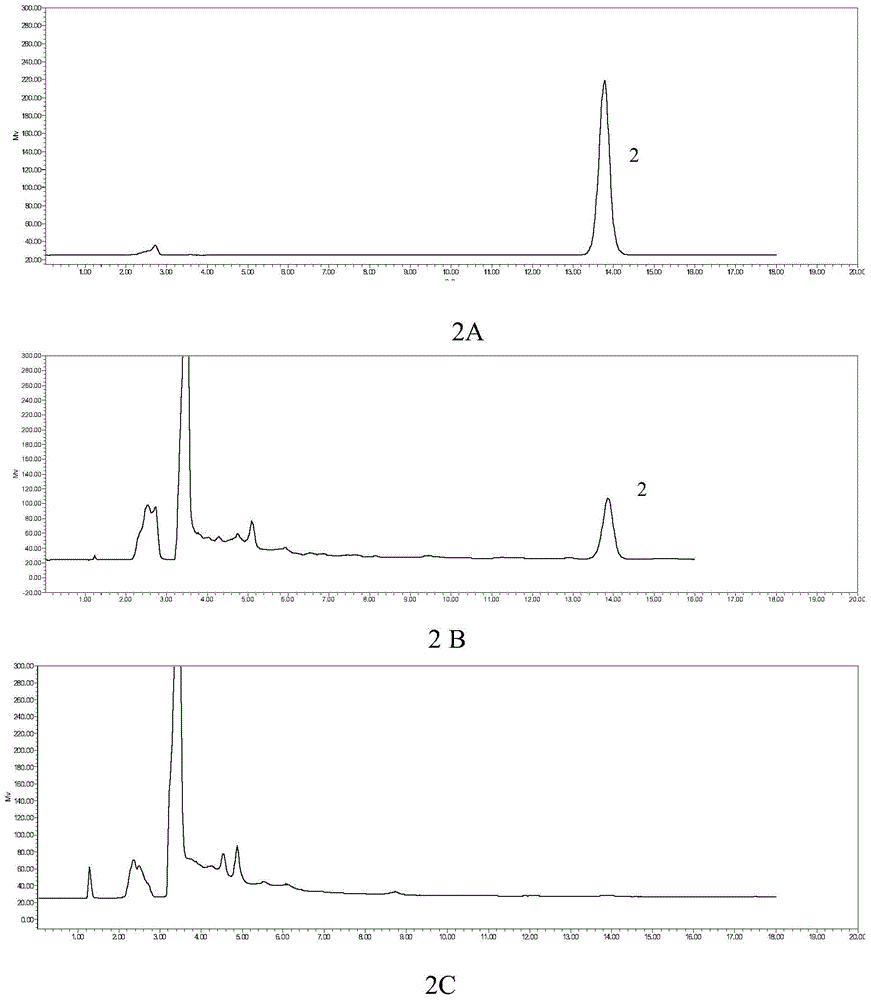
ActiveCN101703611BQuality controllableGood reproducibilityComponent separationDigestive systemContent determinationRepeatability
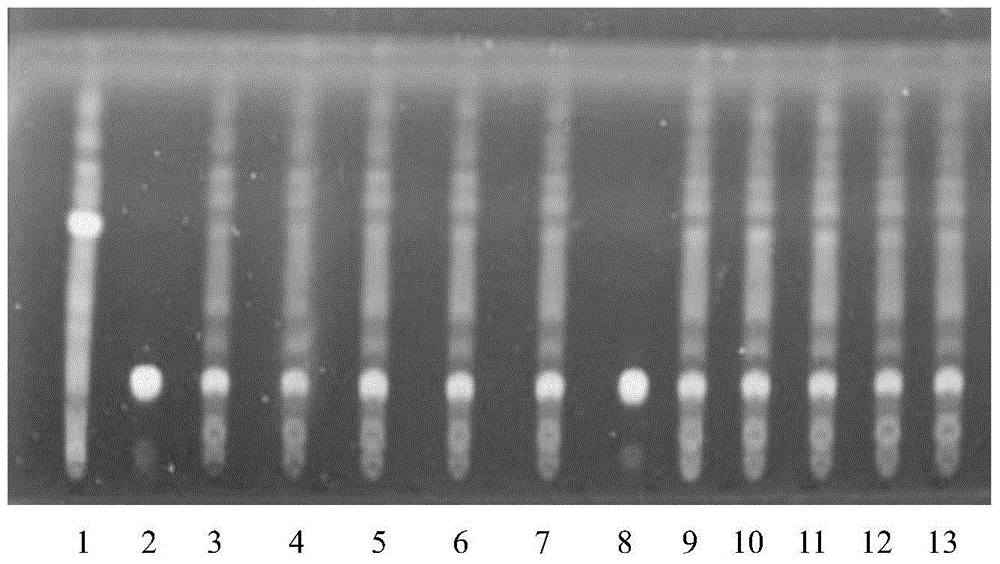
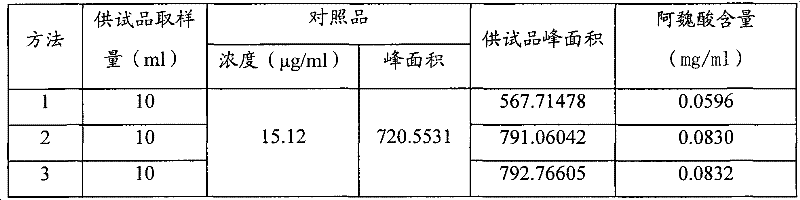
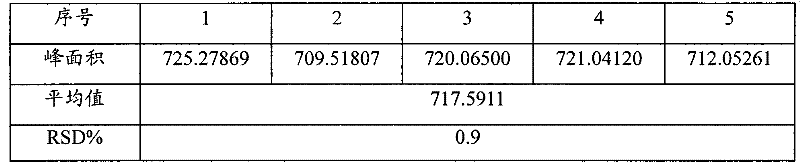
The invention discloses a quality detection method of a Chinese angelica oral liquid for benefiting blood, comprising the following steps of: distinguishing: TLC detection of rhizoma ligustici wallichii and white paeony root; content determination: referencing to high performance liquid chromatography: a. chromatographic condition and system applicability test; b. preparation of a reference substance solution; c. preparation of a test substance solution; and d. a determination method. Counted by ferulic acid (C10H10O4), the content of Chinese angelica and the rhizoma ligustici wallichii in each Chinese angelica oral liquid for benefiting blood is not less than 0.30mg. The method has stability and favorable repeatability and is beneficial to controlling the quality of products.
Owner:GUIYANG XINTIAN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com