Beta glycolipids as immuno-modulators
一种糖脂、免疫相关的技术,应用在抗炎剂、抗病毒剂、抗真菌剂等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] Use of beta-glycolipids for the treatment of immune-mediated colitis
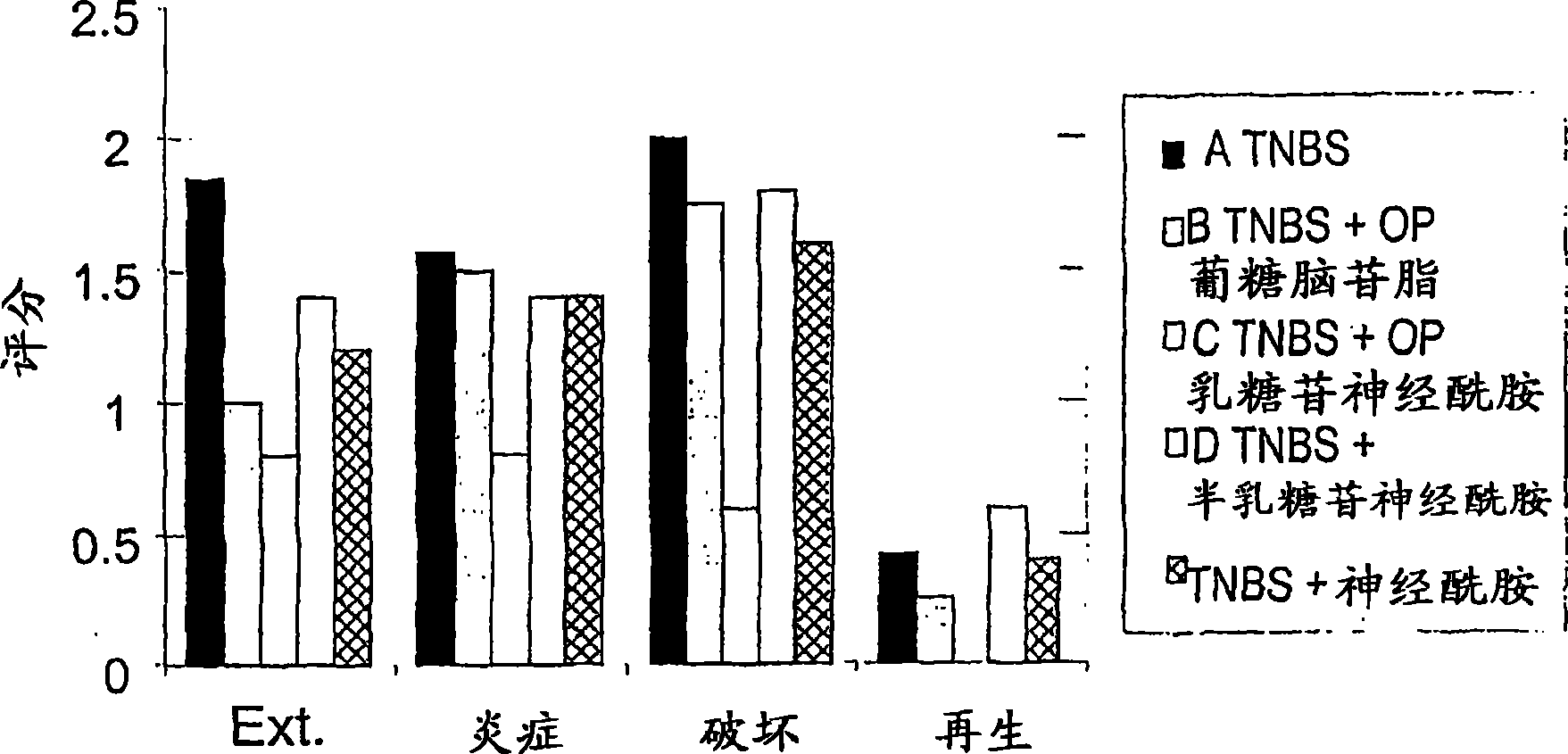
[0222] To determine the effect of administration of β-glycolipids such as β-glucosylceramide (GluC), β-lactosylceramide (LacC) and β-galactosylceramide (GalC) and ceramides on experimental colitis Clinical and immunological effects in the murine model, nine groups of C57B1 mice each consisting of 10 mice were studied. As outlined in Table 1, colitis was induced by intracolonic placement of trinitrobenzenesulfonic acid (TNBS) on days 1 and 5 in groups A-E. Group A mice were fed with conventional diet. Groups B-E mice were orally administered (PO) 15 μg of GluC, LacC, GalC and ceramide each day. Group F-I mice were not treated with TNBS, but were orally administered (PO) 15 μg of GluC, LacC, GalC and ceramide every day, and were used as a control group.
[0223] The visual and microscopic colitis scores of the mice were tracked and recorded. The immunomodulatory effect of GC was determined by FACS ...
Embodiment 2
[0229] Synergistic effect of a mixture of β-glycolipids in the treatment of immune-mediated colitis
[0230] To determine the possible immunomodulatory effects of different β-glycolipids, the effect of a mixture of β-glucocerebroside (GC) and β-lactosylceramide (LC) was tested using a murine model of colitis.
[0231] As outlined in Table 2, 5 groups of mice, each consisting of 10 mice, were studied. Colitis was induced by intracolonic placement of trinitrobenzenesulfonic acid (TNBS) on days 1 and 5 in groups A, B and D. Group A mice were fed with conventional diet. Group B mice received daily oral (PO) 15 μg mixture of β-GluC (GC) and β-LacC (LC), group D received β-LacC only. Groups C and E mice were not treated with TNBS but received 15 μg daily oral (PO) of a mixture of β-GluC and β-LacC or β-LacC alone, respectively, and served as a control group.
[0232] Mice were followed for visual and microscopic colitis scores, as well as for survival and functional status and bo...
Embodiment 3
[0239] Effect of a mixture of β-glycolipids in the treatment of immune-mediated colitis
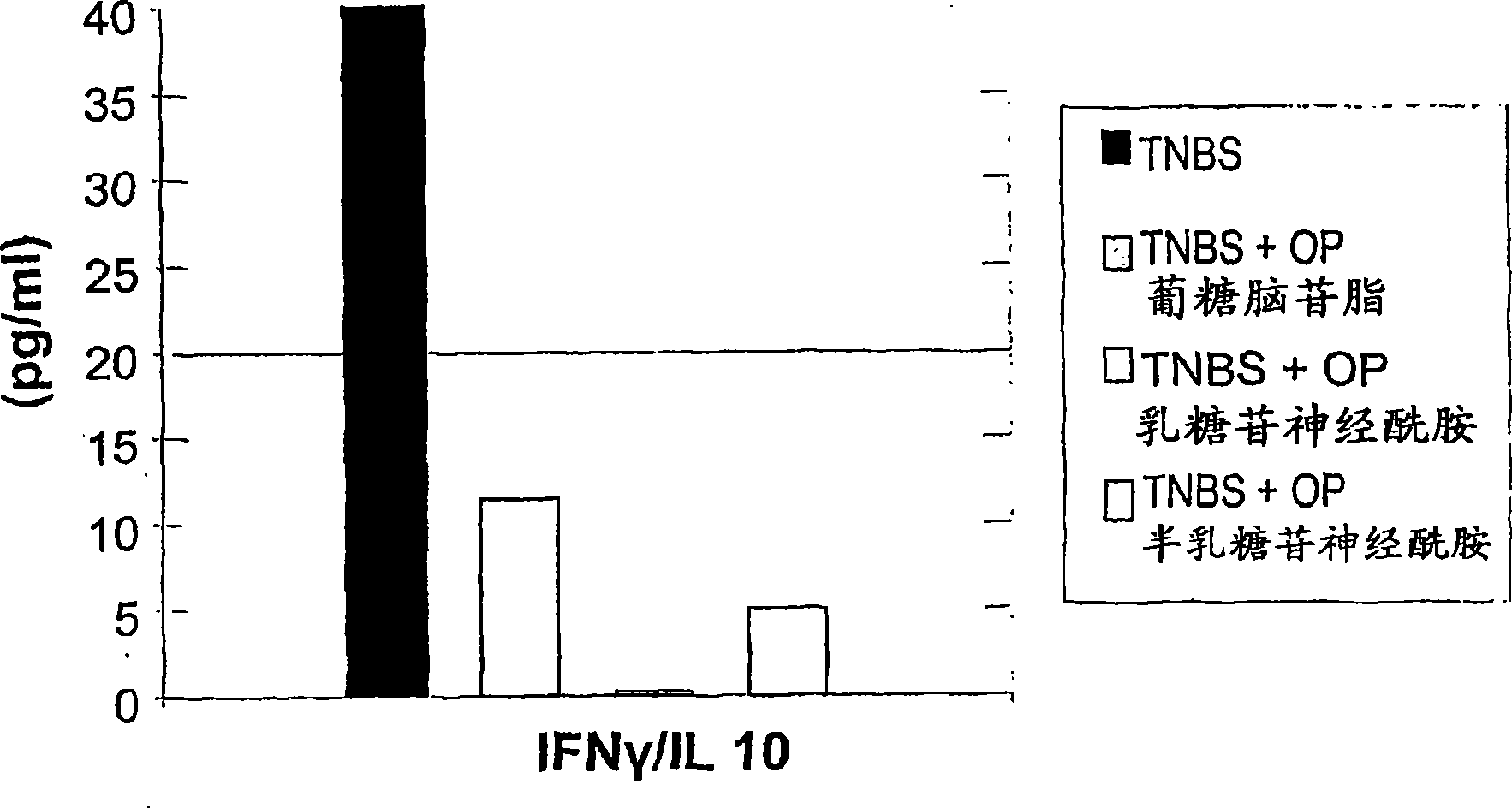
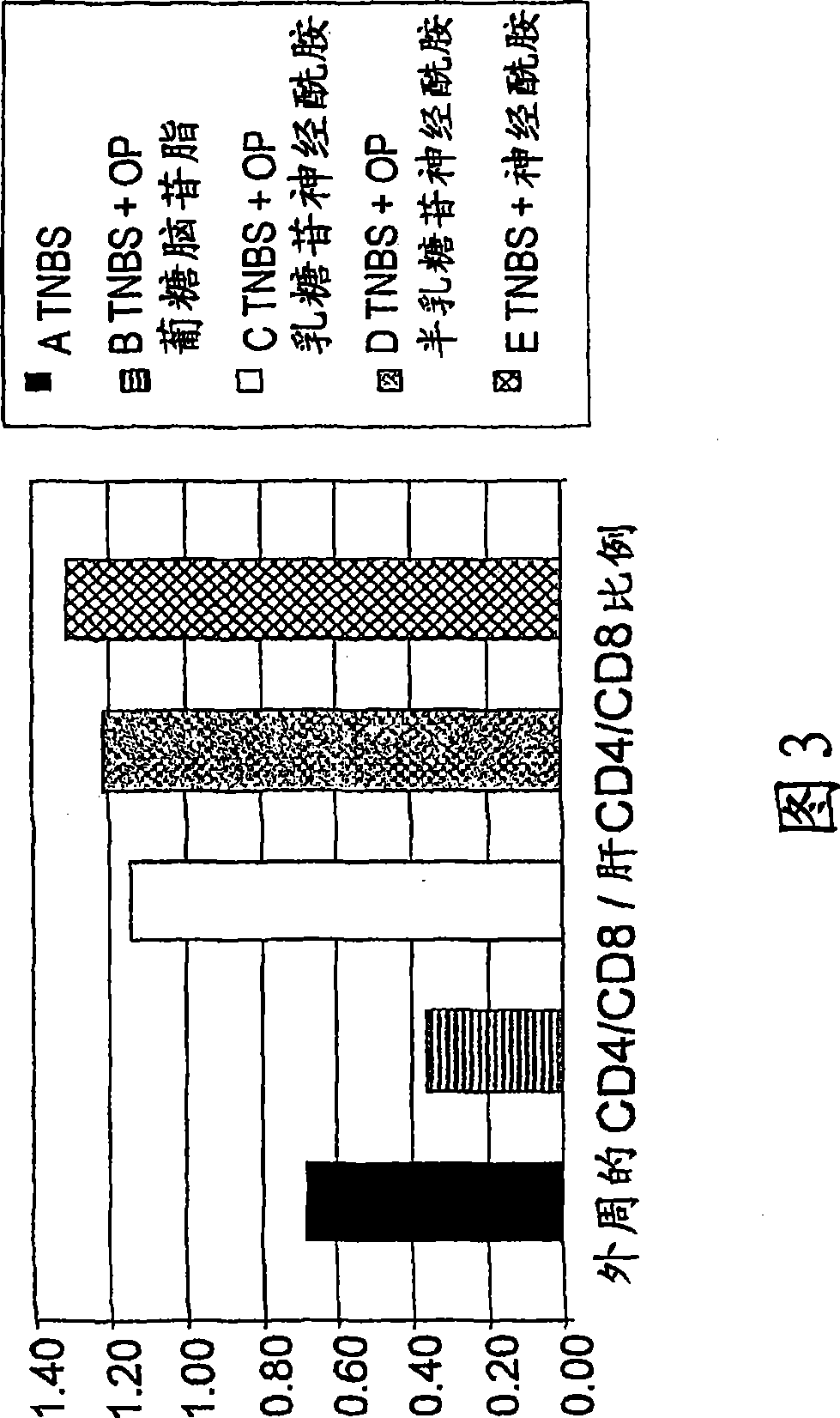
[0240] To further investigate the possible use of β-glycolipids as a drug for colitis, the inventors next determined the effect of the combination of GC and LC on intrahepatic NKT regulatory lymphocytes and lymphocyte capture.
[0241]4 groups of mice were studied. Immune-mediated colitis was induced in all groups by intracolonic placement of trinitrobenzenesulfonic acid (TNBS). Groups B-D were treated by daily administration of glucosylceramide (GC), lactosylceramide (LC) and a combination of GC and LC (1:1 ratio), respectively. Mice in control group A received vehicle only. Mice were evaluated for visual and microscopic colitis scores. The immunomodulatory effects of β-glycolipids were determined by FACS analysis of intrahepatic and intrasplenic lymphocytes for NKT, CD4 and CD8 markers and by measuring serum cytokine levels.
[0242] As shown in Table 4, administration of β-glycolip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com