Peptides of regulatory or accessory proteins of HIV, compositions and the utilization thereof
A technology of polypeptide composition and vaccine composition, which is applied in the field of peptide sequences and can solve problems such as unsatisfactory antigenic determinants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
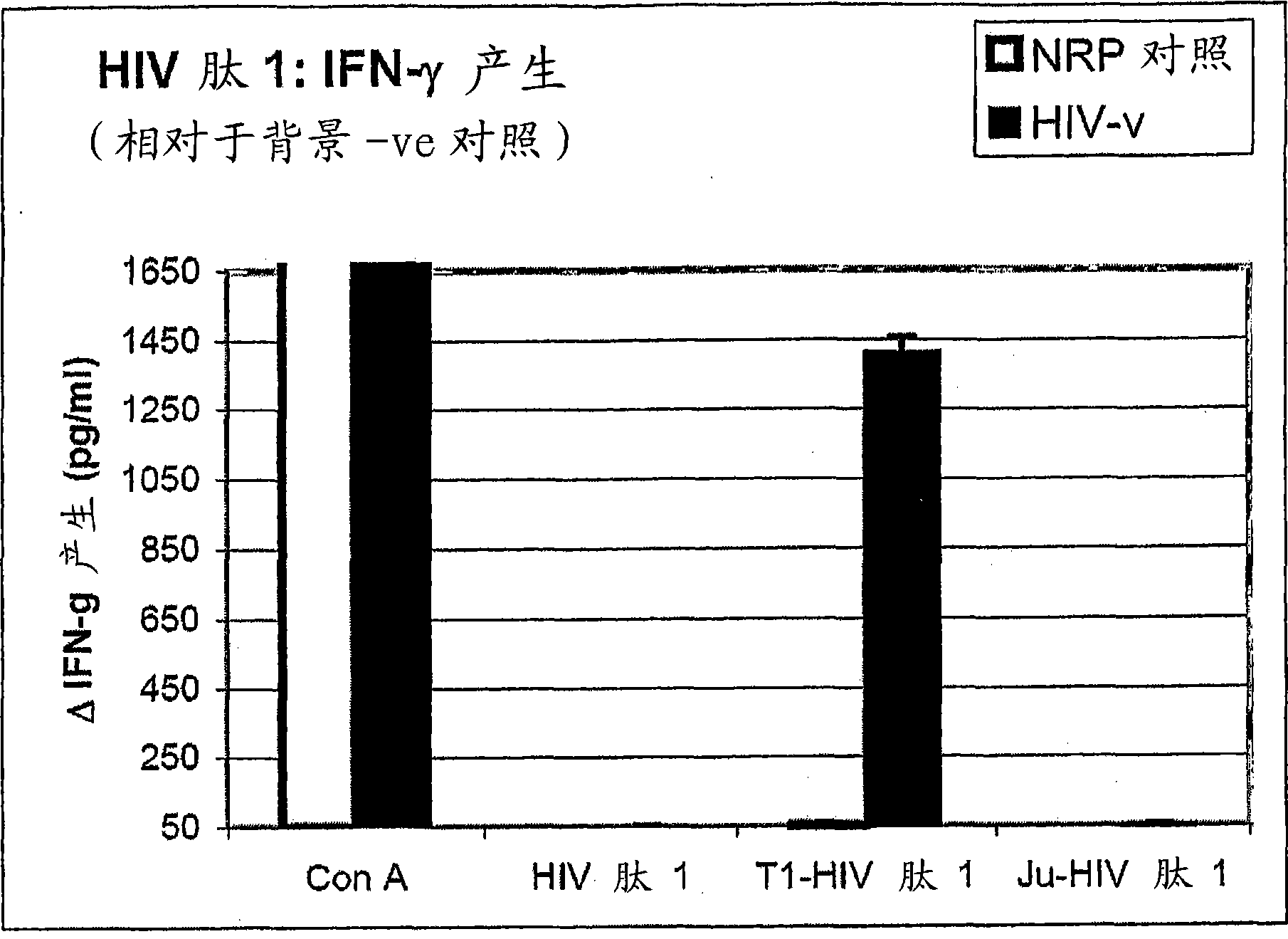
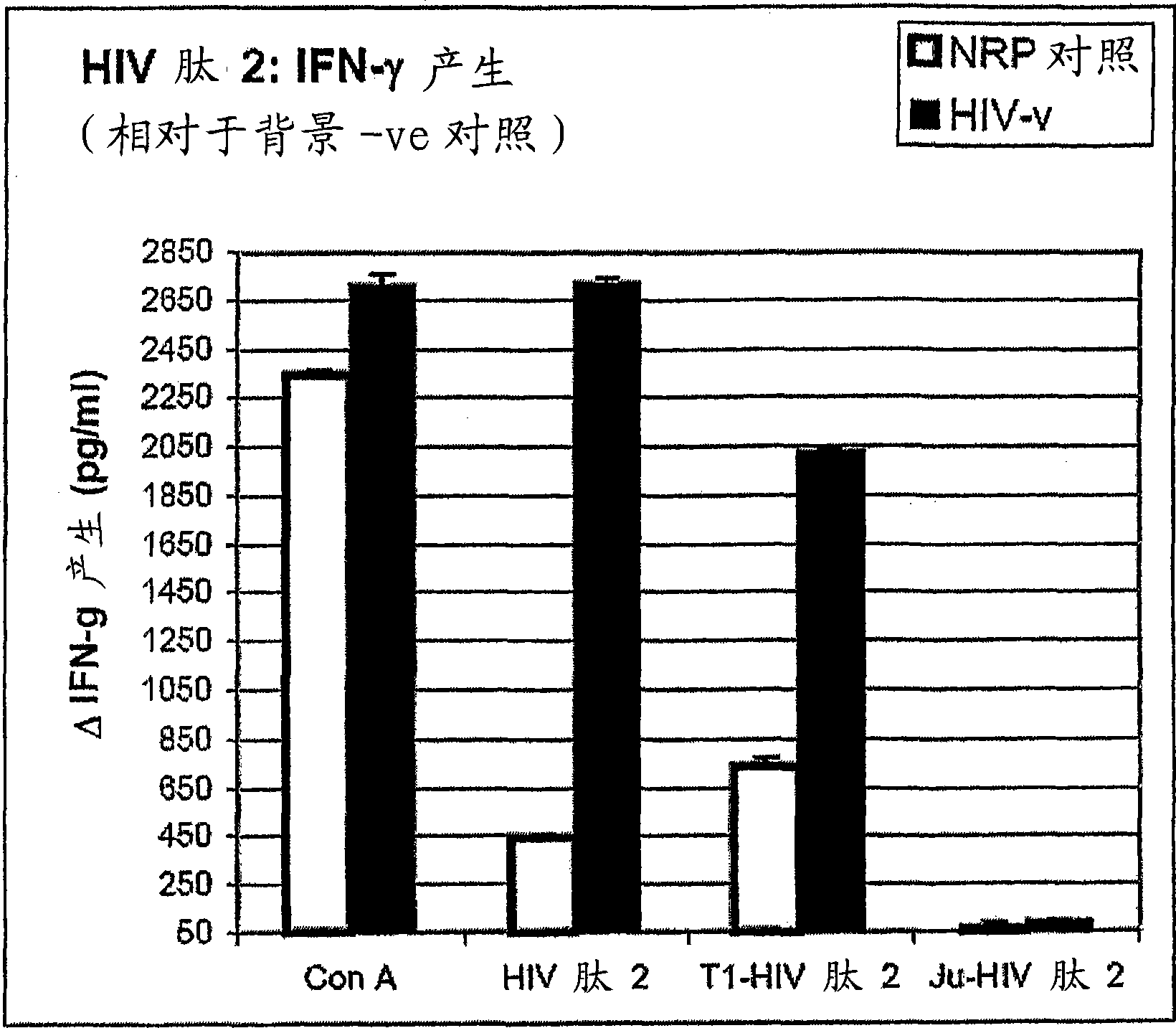
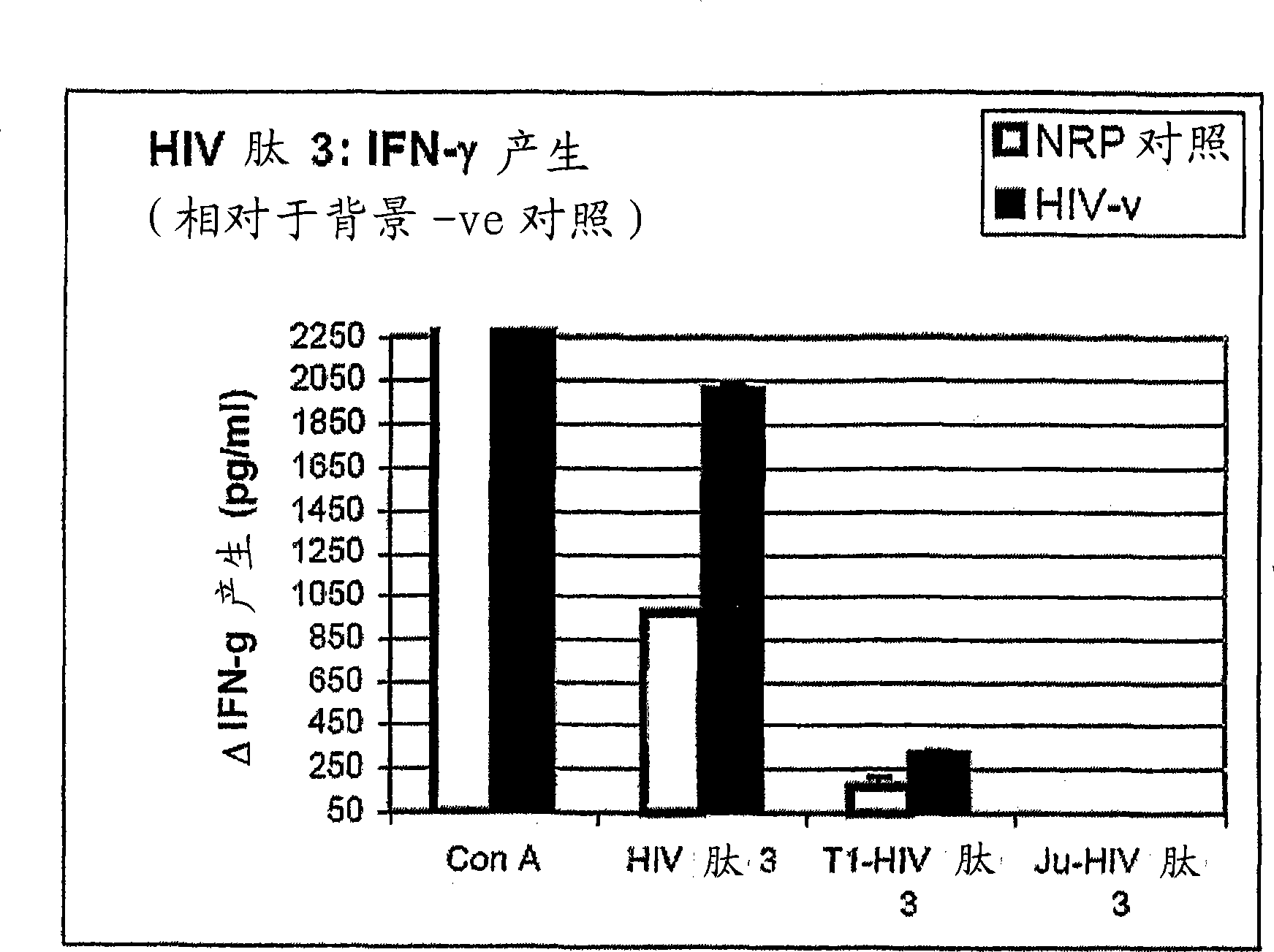
[6158] Embodiment 1, the reactivity of anti-HIV antigen
[6159] The aim of this study was to evaluate the reactivity of the above HIV peptides and their ability to induce specific Th1-type cytokine responses against natively processed and presented HIV proteins in human HLA (HLAA*0201).
[6160] As a background to the experiment, it is useful to understand that Th1 and Th2 responses are defined by the pattern of cytokines produced by the helper T cells they comprise. However, this does not mean that the remaining lymphocytes (T cells and B cells) involved in these specific responses also do not produce cytokines that help drive the characteristic pattern of the responses in which they participate. Thus, Th1 -like responses are characterized by the production of IFN-γ and IL-2, which lead to stimulation of CD8+ CTL responses and associated (in mice) IgG2a antibody responses. IFN-γ responses can be generated by CD4+ helper T cells 1 as well as by CD8+ T cells which also form p...
Embodiment 2
[6228] Example 2. HIV-v Immunization Can Induce Antigen-Specific Responses Against Human Cells Infected by HIV Field Isolates
[6229] The aim of this study was to evaluate whether immunization with identified HIV conserved T-cell polyepitope peptides (HIV-v) could induce antigen-specific responses against human cells infected with HIV field isolates.
[6230] Materials and methods
[6231] Peptides, Viruses and Cell Lines
[6232] The candidate vaccine (HIV-v) used in this study consists of multiple polypeptides (i.e., P1: amino acids 51 to 80 of VPR (SED ID 1); P2: amino acids 142 to 181 of VIF (SED ID 1); ID 2); P3: amino acids 69 to 95 of REV (SED ID 3); P4: amino acids 81 to 123 of NEF (SED ID 4)), these peptides were synthesized by Fmoc chemical method, and re- Suspended in DMSO in PBS (DMSO concentration in the final preparation was less than 10%). Boil-denatured lysozyme (Sigma) was used as a control irrelevant preparation (NRP-v).
[6233] Infectious HIV-1 strains...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com