Method of treating atrophic vaginitis
A technique for vaginal administration and composition, applied in the field of pharmaceutical composition, capable of solving unknown problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
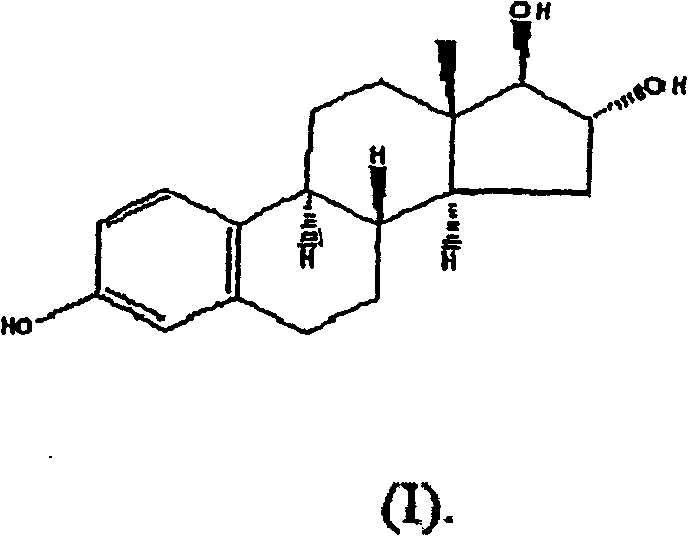
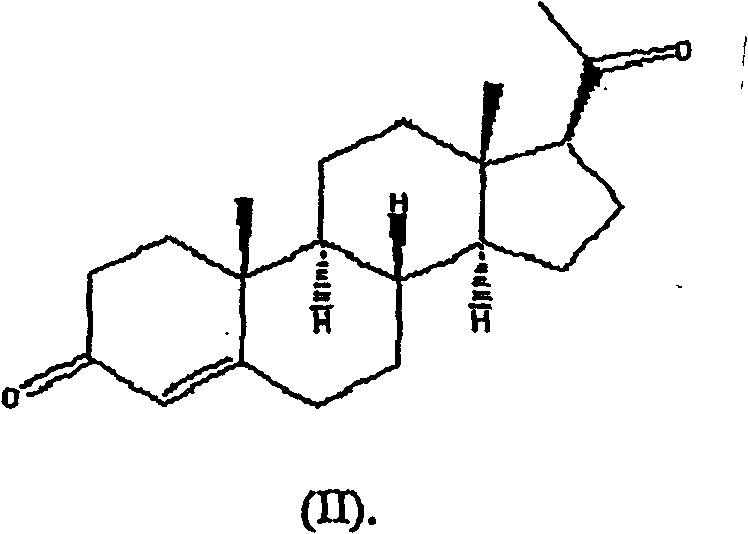
[0091] Example 1: Estrogen / Progesterone Vaginal Suppositories in Atrophic Vaginitis Patients
[0092] This example describes the Phase 1-2, open-label, randomized, single-blind, placebo-controlled study of the safety profile of an estrogen / progesterone vaginal suppository ("JC-001") in postmenopausal patients with atrophic vaginitis. Multiple dose trials.
[0093] The purpose of this study is as follows:
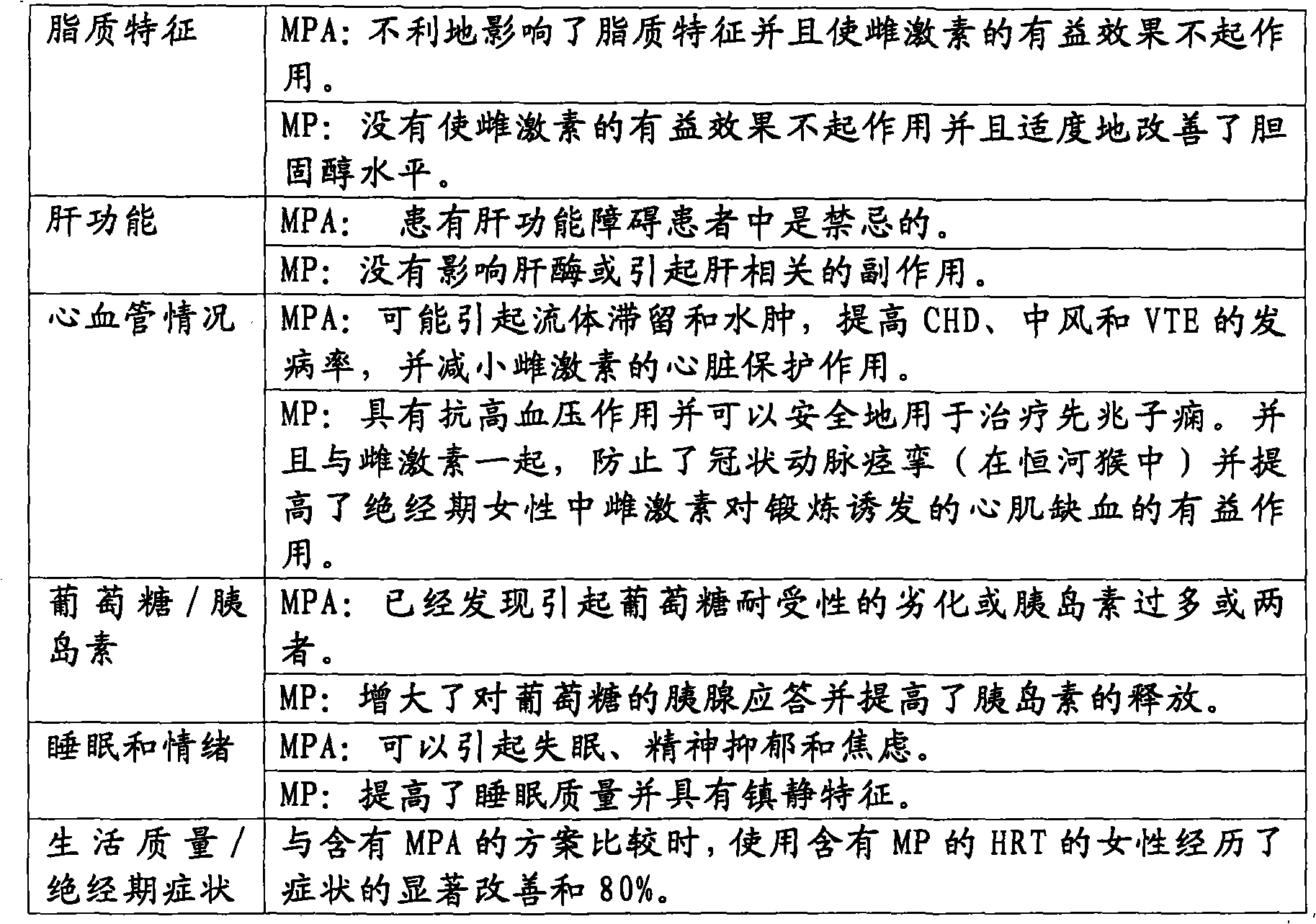
[0094] (1) The purpose of the trial was to determine the efficacy and evaluate the relative safety of placebo, unopposed estrogen, and two combined estrogen-progesterone regimens for the treatment of atrophic vaginitis in postmenopausal women.
[0095] (2) Efficacy, as measured by improvement in vaginal atrophy as measured objectively and subjectively, is to compare the efficacy of the vaginal formulations with each other and with placebo in alleviating the symptoms of atrophic vaginitis. Improved objective measures include measurement of vaginal pH and the presence of va...
Embodiment 2
[0133] Example 2: Pharmaceutical Composition Formulation in Cream Form
[0134] This example provides the formulation of the pharmaceutical composition as a vaginal cream for the treatment of symptoms associated with atrophic vaginitis. Table 6 summarizes the ingredients and their amounts.
[0135] Table 6
[0136] intensity
[0137] For each strength, the total volume per dose is 1gm.
Embodiment 3
[0138] Example 3: Pharmaceutical Composition Formulation in Cream Form
[0139] This example provides the formulation of the pharmaceutical composition as a vaginal cream for the treatment of symptoms associated with atrophic vaginitis. Table 7 summarizes the ingredients and their amounts.
[0140] Table 7
[0141] intensity
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com