Plant source bactericide and artificial synthesis thereof
A botanical fungicide and artificial synthesis technology, which is applied in the field of botanical pesticide preparation to achieve the effects of high yield, simple operation and high inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
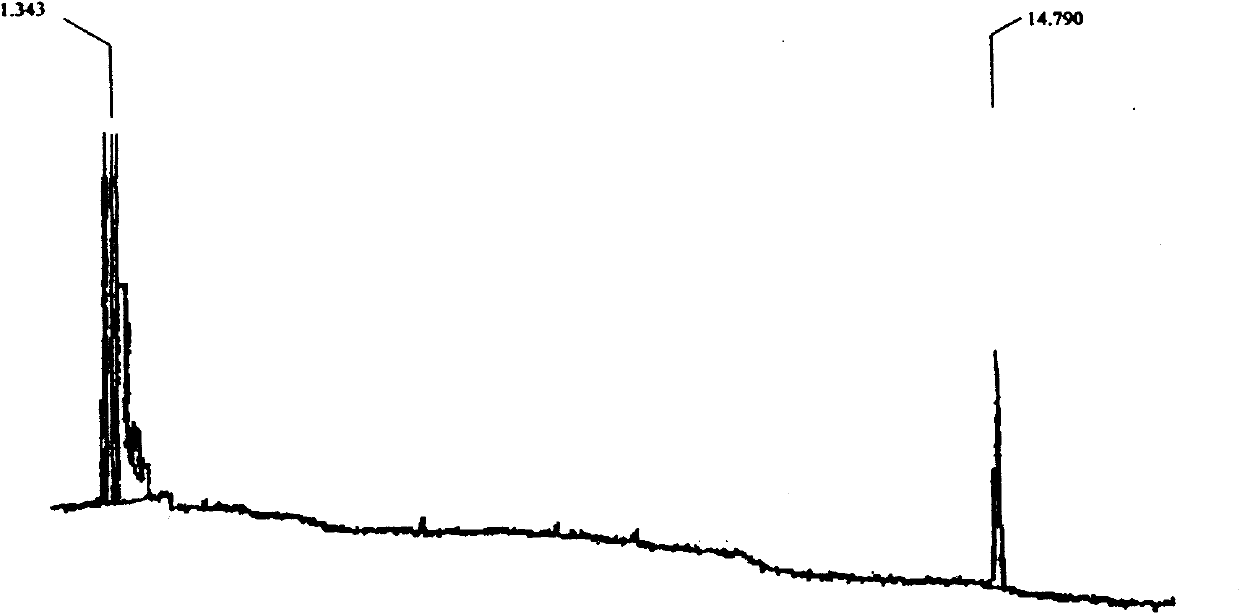
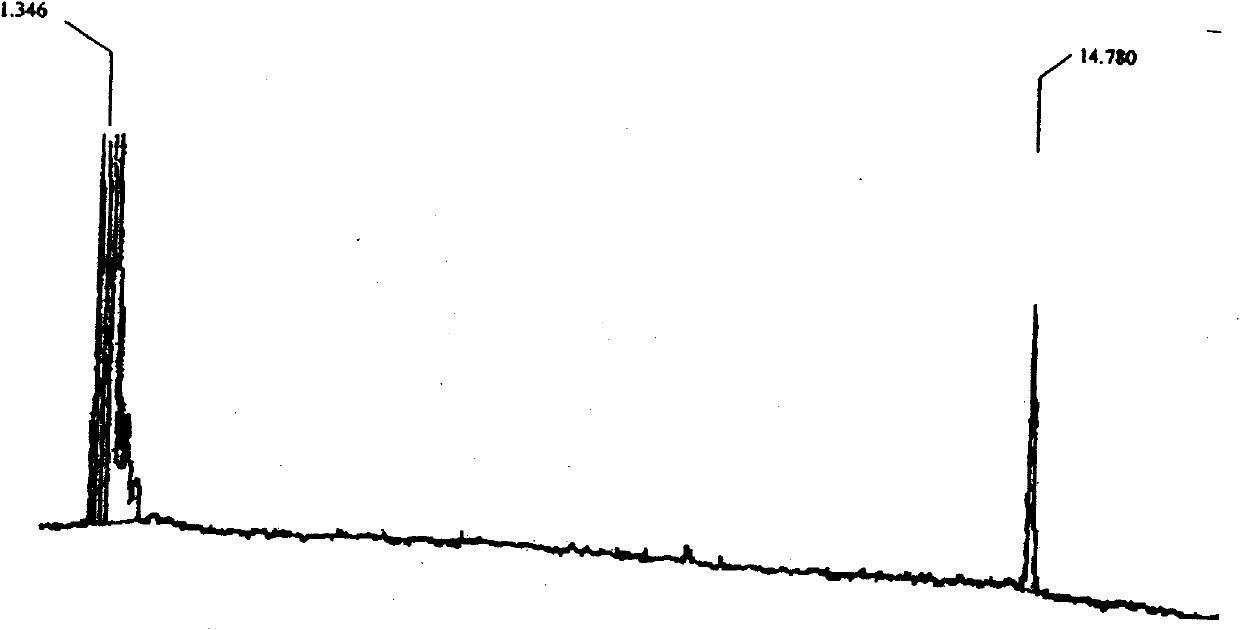
Image
Examples
Embodiment 1
[0045] A plant source fungicide, the fungicide is 2,4-dicyclopentane cyclopentanone, and its molecular formula is C 15 h 20 O, the structural formula is as follows:
[0046] Its synthetic method is: (1) in the 250ml four-neck bottle that stirrer, thermometer, reflux condenser and dropping funnel are housed, add cyclopentanone dropwise in the sodium hydroxide solution of 1mol / L, fully Stir, and then continue to add a catalyst in the reaction solution, and the catalyst is dodecyldimethylbenzylamine bromide. Heating and stirring at a temperature of 80° C. for 1 h until the reaction is complete and the reaction solution turns yellow, the molar ratio of the above-mentioned sodium hydroxide, cyclopentanone and the catalyst is 1: 0.36: 0.01; (2) transfer the above-mentioned reaction solution to the In the liquid funnel, discard the water layer, wash the oil layer with water repeatedly, distill under reduced pressure, collect the fraction at 300-302°C, and obtain a yellow oily liq...
Embodiment 2
[0048] A plant source fungicide, the fungicide is 2,4-dicyclopentane cyclopentanone, and its molecular formula is C 15 h 20 O, the structural formula is as follows:
[0049] Its artificial synthesis method is: (1) in the 250ml four-neck bottle of the dropping funnel that agitator, thermometer, reflux condenser and dropping funnel are housed, add cyclopentanone dropwise in the sodium hydroxide solution of 1mol / L, Fully stir, then continue to add catalyst in reaction liquid, described catalyst is chitosan quaternary ammonium salt. Heating and stirring at a temperature of 120° C. for 2.5 h, until the reaction is complete and the reaction solution turns yellow, the molar ratio of the above-mentioned sodium hydroxide, cyclopentanone and the catalyst is 1: 0.43: 0.01; (2) the above-mentioned reaction solution is transferred to In a separatory funnel, discard the water layer, wash the oil layer repeatedly with water, distill under reduced pressure, collect the fraction at 300-302...
Embodiment 3
[0051] A plant source fungicide, the fungicide is 2,4-dicyclopentane cyclopentanone, and its molecular formula is C 15 h 20 O, the structural formula is as follows:
[0052] Its artificial synthesis method is: (1) in the 250ml four-neck bottle of the dropping funnel that agitator, thermometer, reflux condenser and dropping funnel are housed, add cyclopentanone dropwise in the sodium hydroxide solution of 1mol / L, Stir well, and then continue to add catalyst in the reaction solution, and the catalyst is tri(2-ethyl)hexylamine. Heating and stirring at a temperature of 80°C for 2.5h until the reaction is complete and the reaction solution turns yellow, the molar ratio of the above-mentioned sodium hydroxide, cyclopentanone and the catalyst is 1:0.40:0.01; (2) the above-mentioned reaction solution is transferred to In the separatory funnel, discard the water layer, wash the oil layer repeatedly with water, distill under reduced pressure, collect the fraction at 300-302°C, and o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com