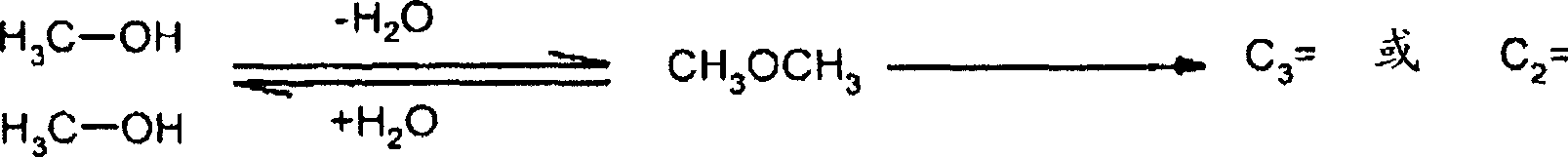Use of a catalyst based on zeolites in the conversion of oxygenates to lower olefins, and associated method
A compound and catalyst technology, applied to the use of zeolite-based catalysts in converting oxygenated compounds into lower olefins and the corresponding fields, can solve the problems of shortened cycle and poor catalyst effectiveness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Modification process of zeolite with iron or iron and cerium
[0066] As pentasil type starting zeolite used was Ammon-MFI type T 4480 from Süd Chemie, Germany.
[0067] Modified with iron:
[0068] Mix 1 kg of initial zeolite with 25 g of FeCl in a ball mill at room temperature 2 *4H 2 O grind together for an hour. The mixture was heated in a chamber furnace in air from room temperature to 550° C. within 3 hours and kept there for 6 hours. After cooling the mixture obtained with 1 wt% Fe content (as Fe 2 o 3 Calculated) modified zeolite.
[0069] Modified with iron and cerium:
[0070] Mix 1 kg of initial zeolite with 25 g of FeCl in a ball mill at room temperature 2 *4H 2 O and 11.4 g CeCl 3 *7H 2 O grind together for an hour. The mixture was heated in a chamber furnace in air from room temperature to 550° C. within 3 hours and kept there for 6 hours. After cooling the mixture obtained with 1 wt% Fe content (as Fe 2 o 3 Calculation) and 0.5...
Embodiment 2
[0071] Example 2: Detection of the improved zeolite obtained from Example 1 within the scope of the invention Hydrothermal stability (compared to zeolites not modified with metals)
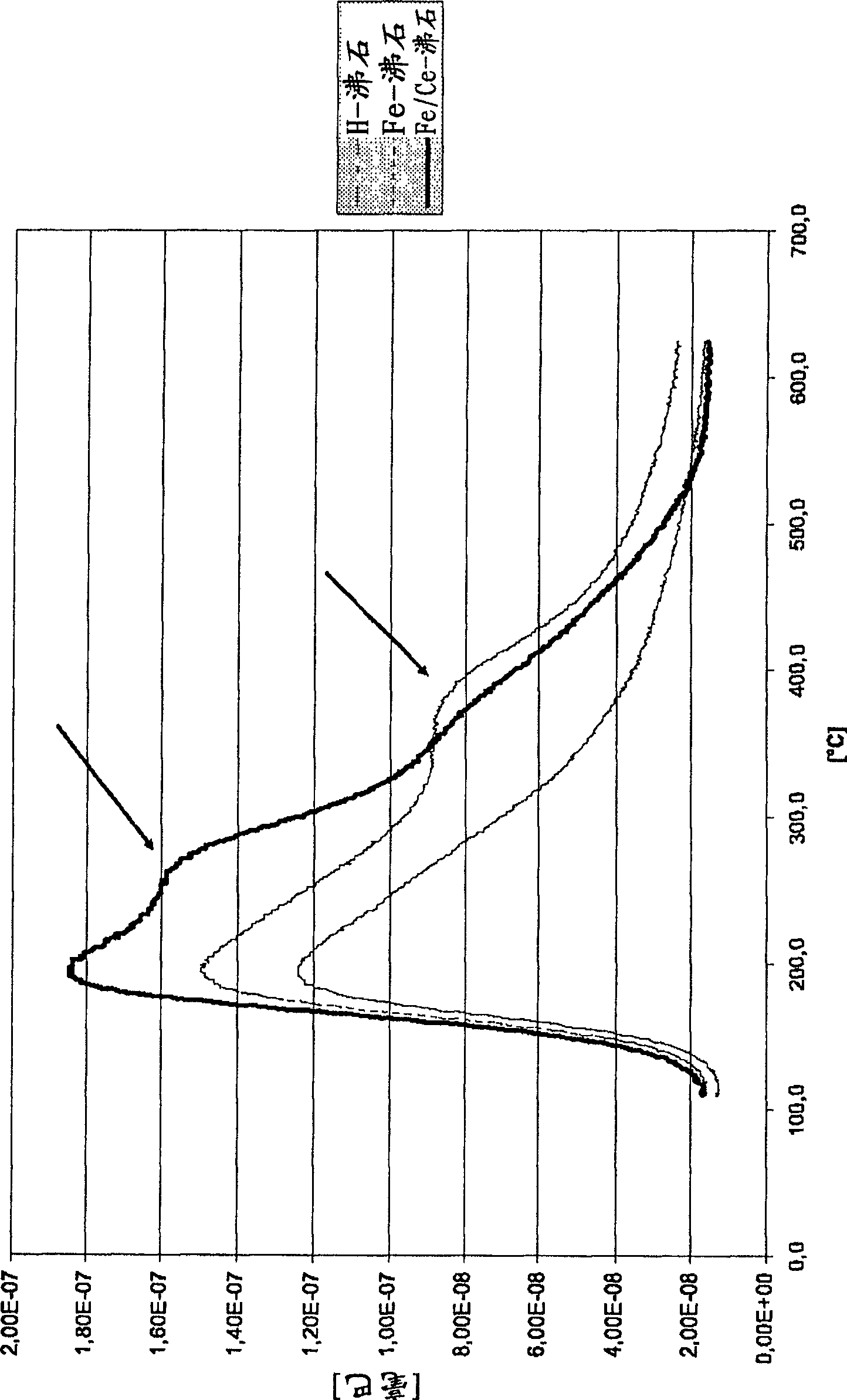
[0072] Depend on figure 1 It can clearly be seen that better results are achieved with the Fe-modified zeolite than with the same zeolite in its H-form, ie there is a better hydrothermal stability. Especially in the case of Fe-modified zeolites there is an adsorption band in the range of approximately 380 to 400° C. (indicated by arrows), whereas it is absent in the case of H-zeolites. However, the band at the higher temperature is a measure for the hydrothermal stability of the zeolite, since the zeolite is apparently still capable of adsorbing NH at the elevated temperature 3 .
[0073] Even better are the results in the case of using zeolites modified not only with Fe but also with Ce. Here, already at approximately 280° C., a distinct (indicated by an arrow) adsorption band can be seen....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com