Ibuprofen percutaneous release patch and preparation method thereof
A technology of patch and transdermal penetration enhancer, which is applied in the field of medicine, can solve the problems of short biological half-life of ibuprofen, frequent medication, kidney damage, etc., achieve good sweat resistance and repeated peeling, and overcome insufficient dosage. Determination, the effect of a large amount of accumulated transdermal release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Combine IBU, SIS, medical grade terpene resin, compound transdermal accelerator (72-6:1, 2-propanediol mass ratio=1:1) and compound antioxidant (antioxidant 1010: antioxidant 264 Mass ratio=2: 1) is to carry out back-up by mass percent respectively at 10%, 50%, 33%, 5% and 2%; The consumption of organic solvent (cyclohexane: ethyl acetate mass ratio=3: 1) is 20ml / g IBU;
[0027] 2) Add the SIS and tackifying terpene resin into the organic solvent, add the drug ibuprofen, compound transdermal penetration enhancer and compound antioxidant after complete swelling, stir evenly, and use a film coater to coat after removing air bubbles by ultrasonic waves Put it on the polytetrafluoroethylene film of the anti-adhesive layer, dry it naturally, put it in a vacuum drying oven at 50 ° C for 1.5 hours, remove the solvent completely, obtain the drug storage layer, and then cover the backing layer aluminum foil-polyethylene composite film on the on the drug storage layer and tr...
Embodiment 2
[0029] 1) Combine IBU, SIS, medical grade terpene resin, compound transdermal accelerator (72-6:1, 2-propanediol mass ratio=1:0.1) and compound antioxidant (antioxidant 1010: antioxidant 264 Mass ratio=1: 5) is to carry out back-up respectively by mass percent as 15%, 28.5%, 54%, 1% and 1.5%; The consumption of organic solvent (cyclohexane: ethyl acetate mass ratio=1: 1) is 20ml / g IBU;
[0030] 2) Same as step 2) in Example 1.
Embodiment 3
[0032] 1) Combine IBU, SIS, medical grade terpene resin, compound transdermal accelerator (72-6:1, 2-propanediol mass ratio=1:0.5) and compound antioxidant (antioxidant 1010: antioxidant 264 Mass ratio=1: 0.1) is carried out back-up according to mass percentage respectively at 5%, 65%, 25%, 4% and 1%; The consumption of organic solvent (cyclohexane: ethyl acetate mass ratio=1: 0.1) is 20ml / g IBU;
[0033] 2) Same as step 2) in Example 1.
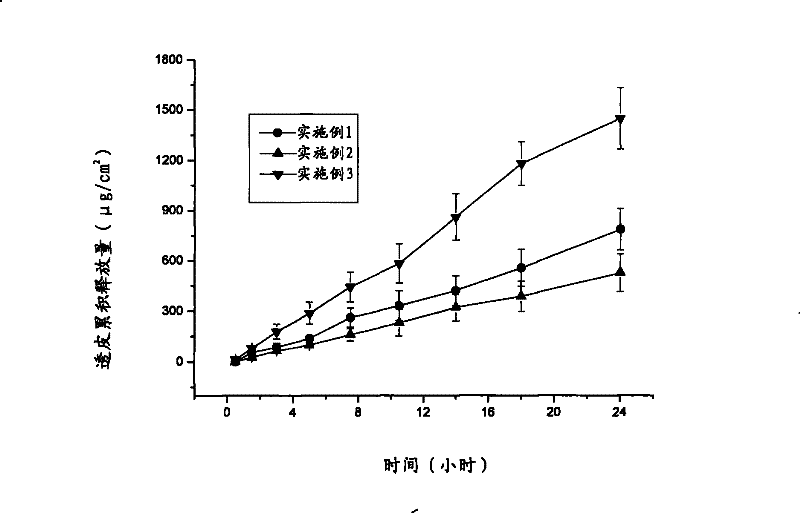
[0034] 1. Drug release test
[0035] Get the ibuprofen transdermal release patch prepared in embodiment 1-3 and carry out in vitro guinea pig permeability test, concrete steps are as follows:
[0036] Fix the pretreated guinea pig skin between the supply chamber and the receiving chamber of the Franz diffusion cell, the cuticle faces the supply chamber, and the supply liquid is close to the cuticle, and a freshly prepared phosphate buffer solution with a pH of 7.4 is added to the receiving chamber When it is full, the dermis side is in f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com