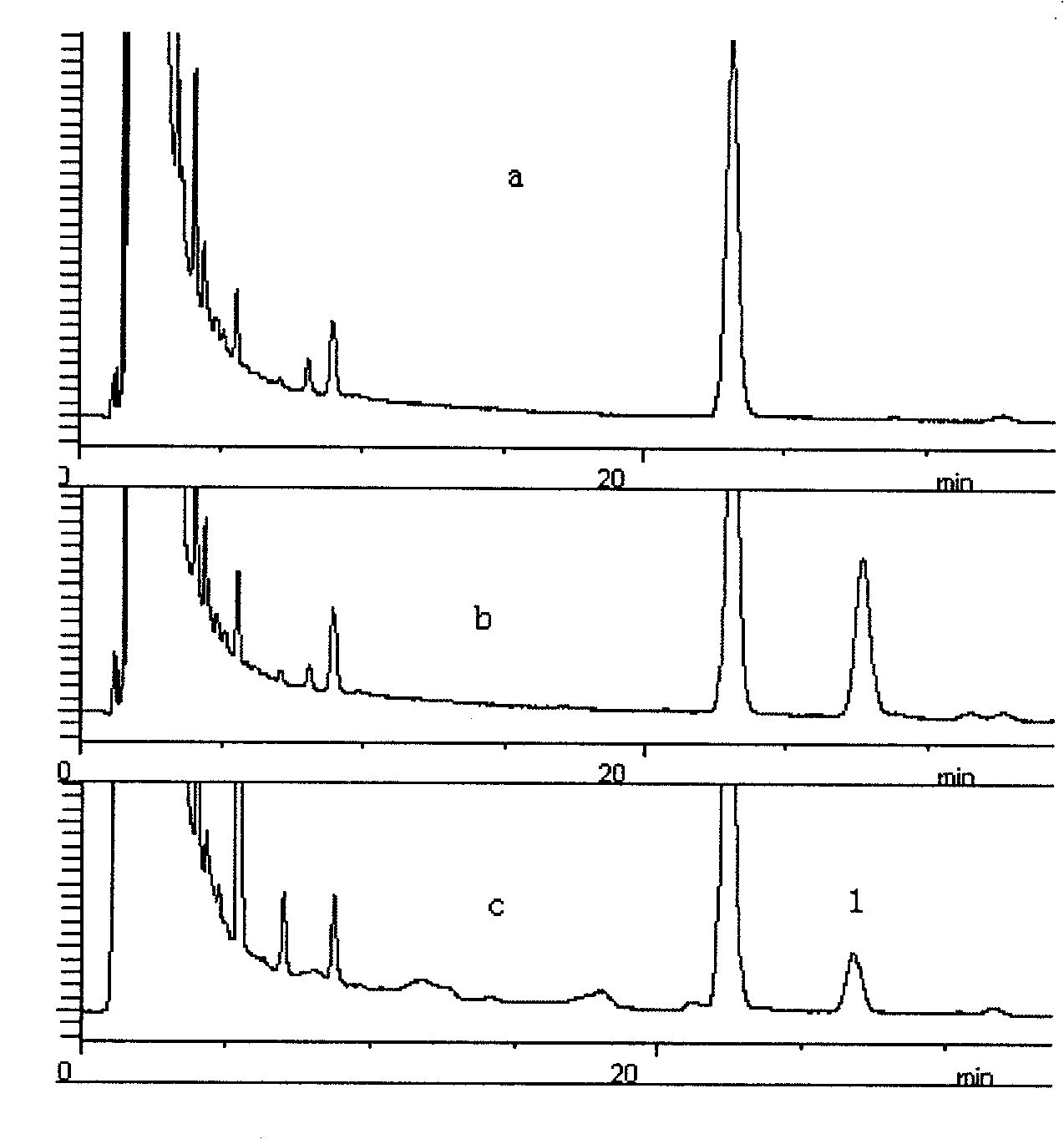
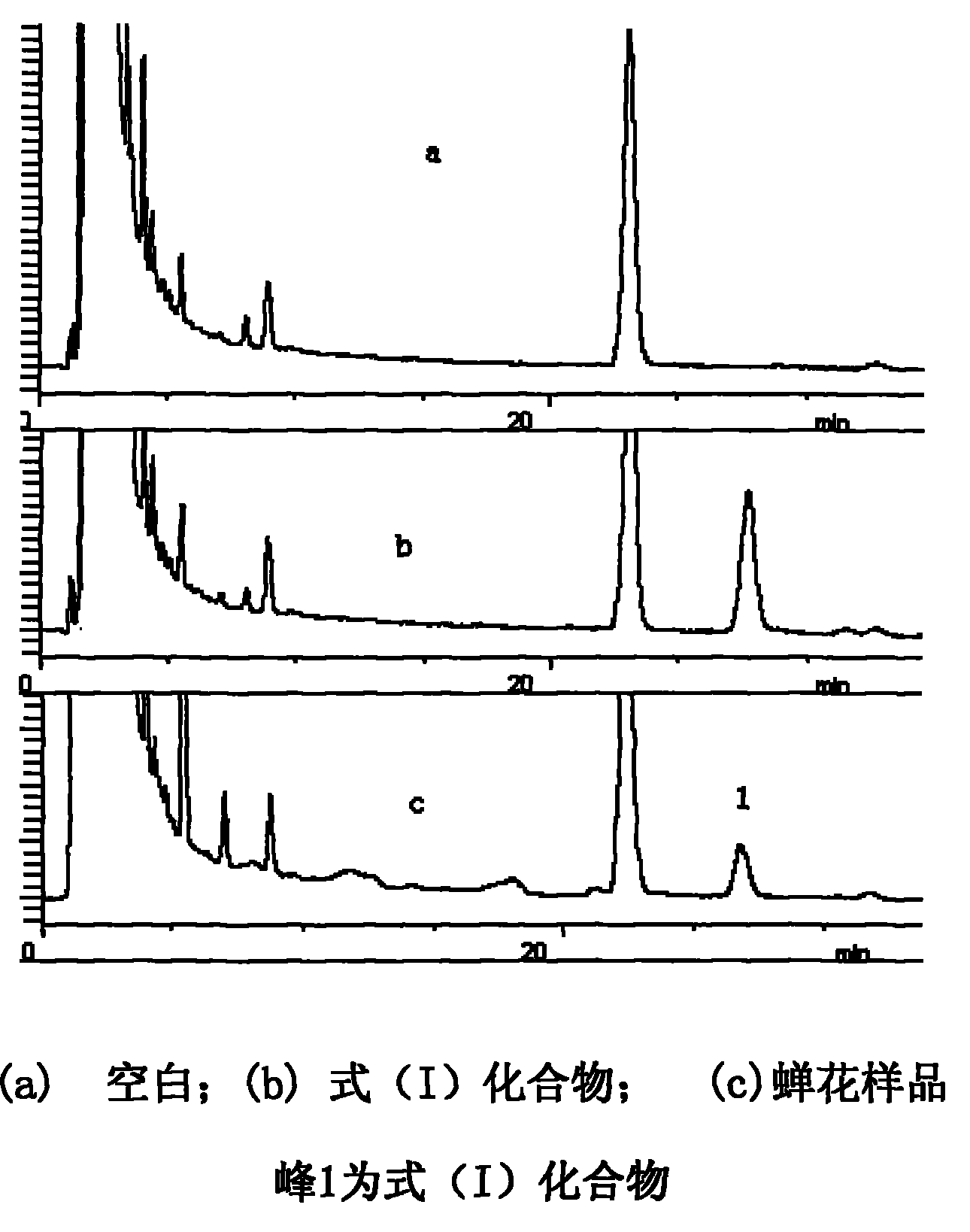
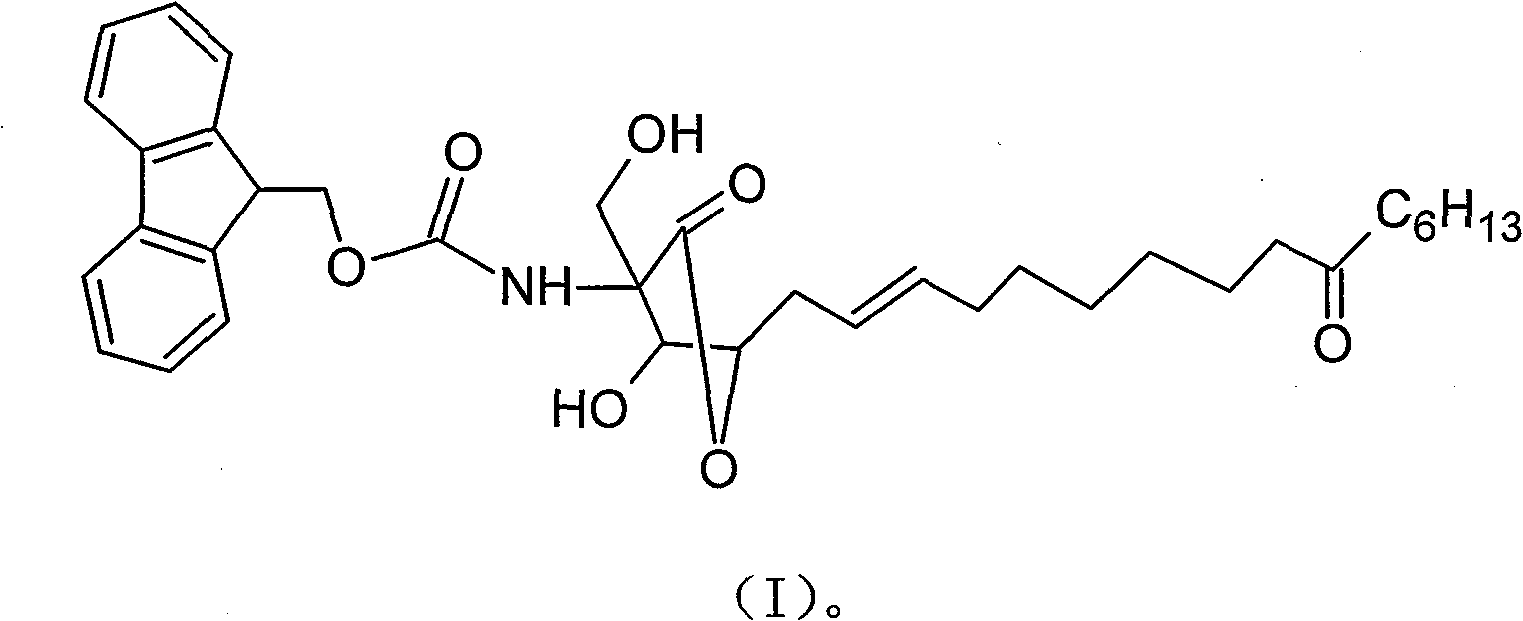
Multi-shell rhzomorph derivative, preparation and uses thereof
A technology of myriocin and its derivatives, which is applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of low content of myriocin, cumbersome operation, low sensitivity, etc., and achieve the effect of reliable measurement results.
Inactive Publication Date: 2011-01-05
CHONGQING ACAD OF CHINESE MATERIA MEDICA
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The disadvantage of this method is that: myriocin is a compound without chromophoric groups, and the detection wavelength needs to be selected at the ultraviolet end, and the determination of the ultraviolet end is subject to much interference and the sensitivity is not high; the chemical composition of the sample is complex, multi-sphere The content of chitosin is low, and it needs to be separated and purified before it can be analyzed by HPLC, which is cumbersome to operate
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a myriocin derivative and a preparation method thereof. The preparation method comprises the following steps: 9-fluorenylmethoxycarbonyl chloride is added to myriocin under the condition that pyridine and tetrahydrofuran solution provide a reaction environment, the 9-fluorenylmethoxycarbonyl chloride reacts with the myriocin to generate the myriocin derivative, the derivative introduces a group with ultraviolet characteristic absorption peaks to amino groups of the myriocin, and quantitative analysis can be carried out by high performance liquid chromatography, thus further quantitatively characterizing the myriocin. The method has reliable and accurate determination results, and can be used for determining myriocin content of traditional Chinese herbal medicines such as cicada fungus, Chinese caterpillar fungus and the like, determining preparations containing the cicada fungus or the Chinese caterpillar fungus and extracts thereof, and determining the myriocin content of biological samples.
Description
A kind of myriocin derivative and its preparation method and application technical field The present invention relates to a myriocin derivative, in particular to a myriocin derivative introduced into the amino group with a characteristic ultraviolet absorption peak group and a preparation method thereof, and also relates to the quantitative determination of the derivative of the myriocin Application of contocin in biological products. Background technique Myriocin has a variety of pharmacological effects. Studies by Fujita et al. have shown that myriocin has a significant two-way immunoregulatory effect, can block the pathway below the interleukin-2 receptor, inhibit the activity of serine palmitoyltransferase, and thus specifically inhibit the proliferation of T cells; Myriocins have a strong inhibitory activity on the induction of the same kind of cell barrier T cells, which is 10 to 100 times stronger than cyclosporin A in these aspects; in the mixed reaction of the sa...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D307/33G01N30/02
Inventor 毛先兵余佳文朱华李徐红娟陈仕江马开森钟国跃
Owner CHONGQING ACAD OF CHINESE MATERIA MEDICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com