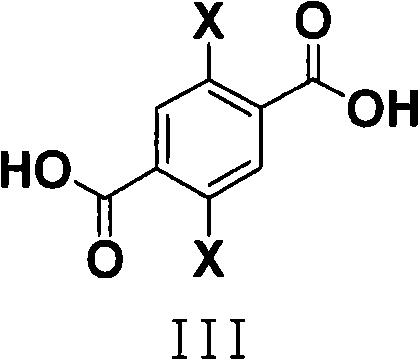
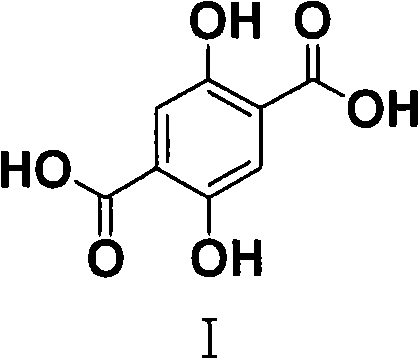
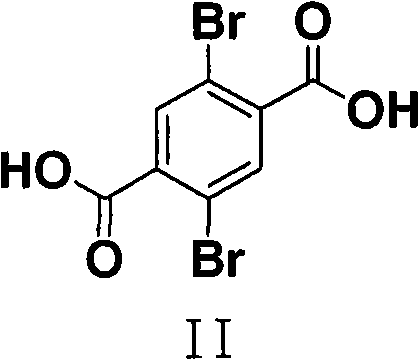
Process for the synthesis of 2,5-dihydroxyterephthalic acid
A technology of hydroxyterephthalic acid and dialkoxy terephthalic acid, which is applied in the field of synthesizing 2,5-dihydroxyterephthalic acid, which can solve the problems of decreased productivity, long reaction time, conversion rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0055] The invention is further defined in the following examples. These examples, while indicating preferred embodiments of the invention, are given by way of illustration only and do not limit the scope of the appended claims. From the above discussion and these Examples, the essential characteristics of this invention can be clearly understood, and without departing from the spirit and scope of the invention, it can be modified to adapt it to various usages and conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com