Method for separating enantiomers of rotigotine and trihexyphenidyl
An enantiomer, trihexyphenidyl technology, applied in the field of capillary electrophoresis chiral separation, achieves the effects of less sample consumption, low ultraviolet absorption background, and high separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Prepare running buffer and samples
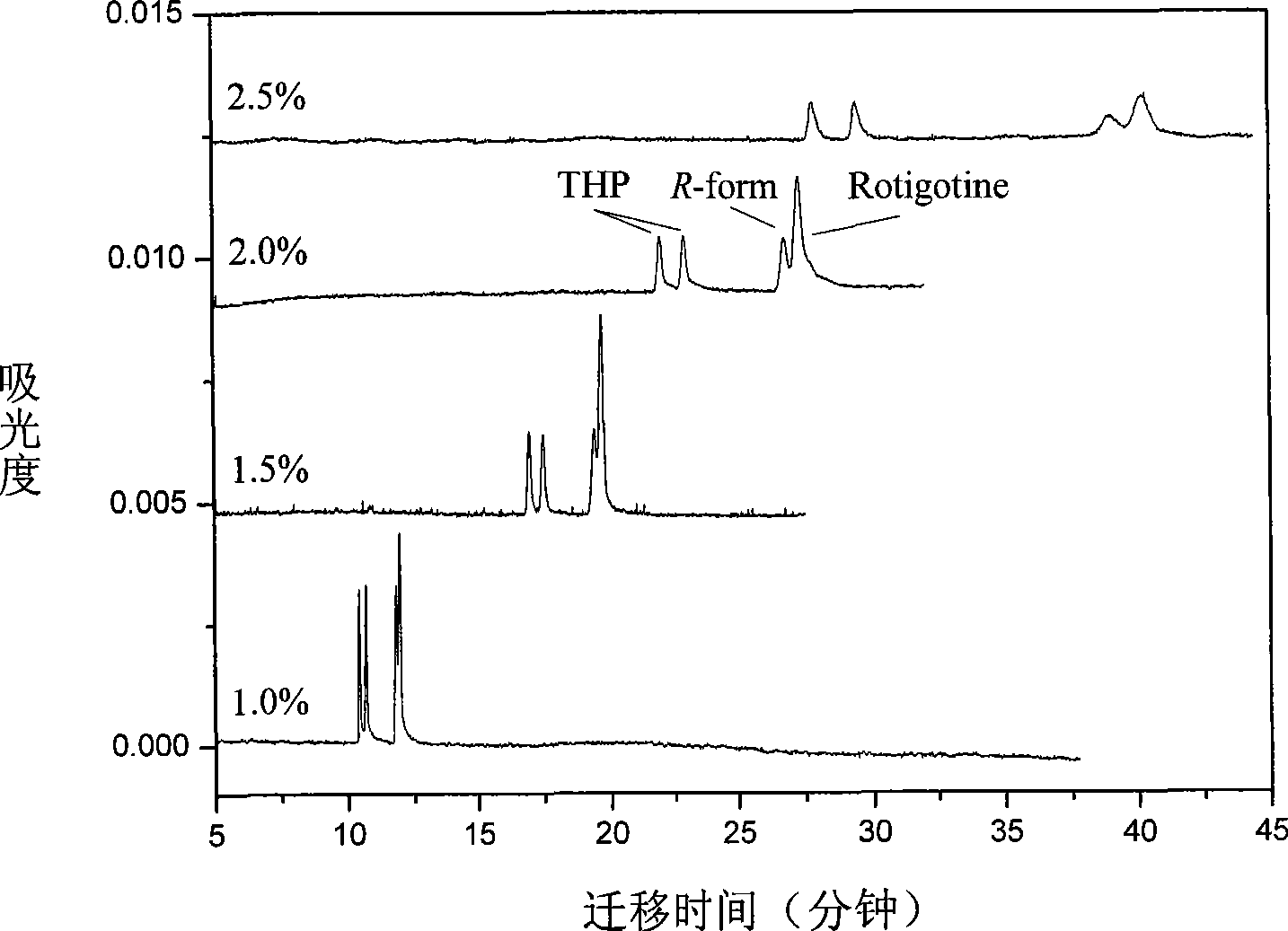
[0053] Using ultrapure water as a solvent, prepare an aqueous solution of sodium dihydrogen phosphate with a concentration of 10 mmol / L as the background electrolyte. Slowly add phosphoric acid with a concentration of 1 mol / L dropwise into the prepared sodium dihydrogen phosphate aqueous solution to adjust the pH value of the buffer solution to 4. Sulfonated dextran with a molecular weight of 1,000,000 was added to the above buffer solution at a ratio of 2.0% (w / v), dissolved, filtered through a 0.22 μm filter membrane, and ultrasonicated for 5 minutes. Dissolve rotigotine and trihexyphenidyl in ultrapure water to obtain stock samples with a concentration of 10 mmol / L, and store them at 4°C. During sample injection, the stock sample was diluted with running buffer to obtain the sample to be separated with a concentration of 0.1 mmol / L.
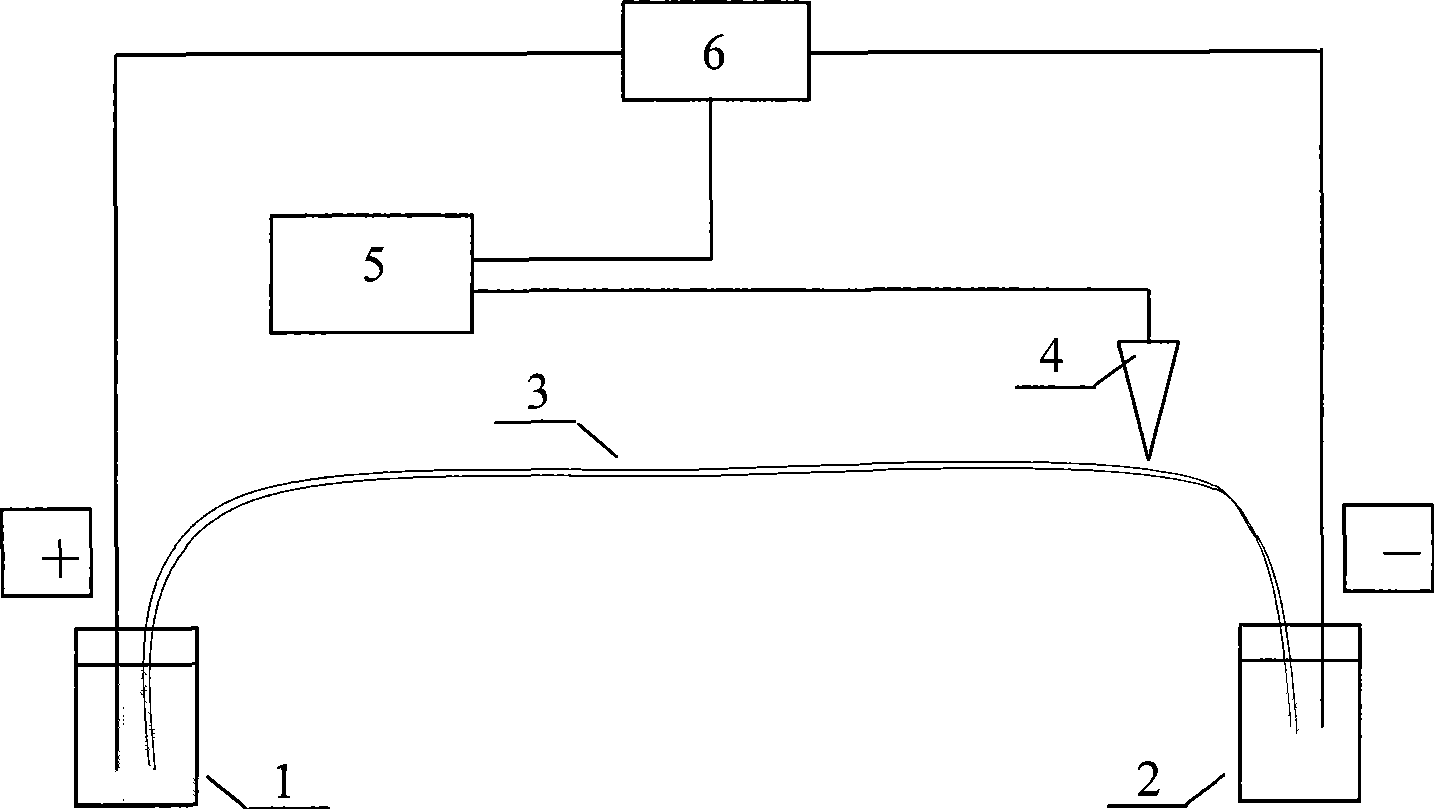
[0054] 2. Instrument Preparation
[0055] The separation process was carried out in an unco...
Embodiment 2
[0063] 1. Prepare running buffer and samples.
[0064] Using ultrapure water as a solvent, prepare an aqueous solution of sodium dihydrogen phosphate with a concentration of 10 mmol / L as the background electrolyte. Slowly add phosphoric acid with a concentration of 1 mol / L dropwise into the prepared sodium dihydrogen phosphate aqueous solution to adjust the pH value of the buffer solution to 2.5. Sulfonated dextran with a molecular weight of 1,000,000 was added to the above buffer solution at a ratio of 2.0% (w / v), dissolved, filtered through a 0.22 μm filter membrane, and ultrasonicated for 5 minutes. Dissolve rotigotine and trihexyphenidyl in ultrapure water to obtain stock samples with a concentration of 10 mmol / L, and store them at 4°C. During sample injection, the stock sample was diluted with running buffer to obtain the sample to be separated with a concentration of 0.1 mmol / L.
[0065] 2. Instrument Preparation
[0066] The separation process was carried out in an u...
Embodiment 3
[0074] 1. Prepare running buffer and samples.
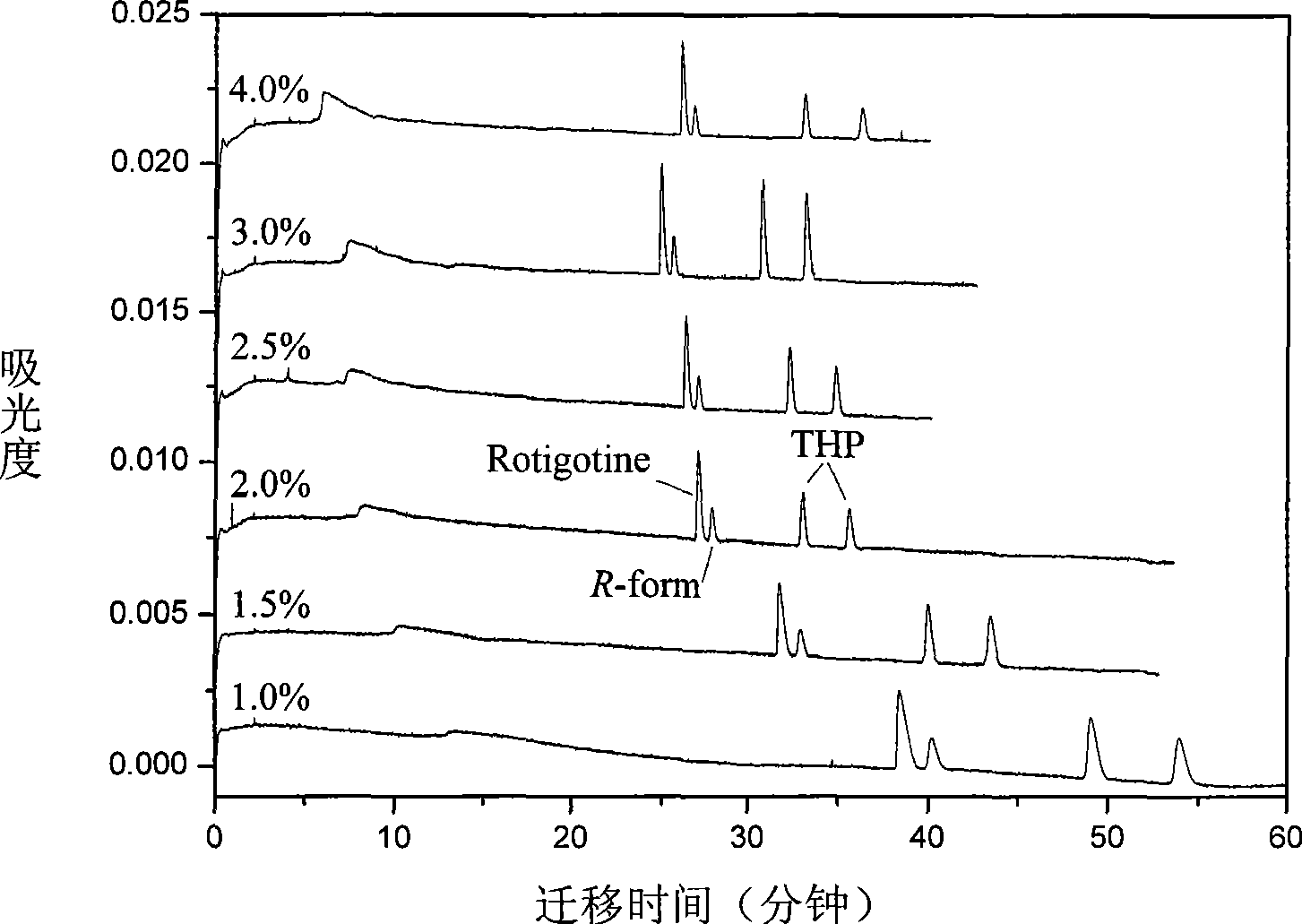
[0075] Using ultrapure water as a solvent, prepare an aqueous solution of sodium dihydrogen phosphate with a concentration of 10 mmol / L as the background electrolyte. Slowly add phosphoric acid with a concentration of 1 mol / L dropwise into the prepared sodium dihydrogen phosphate aqueous solution to adjust the pH value of the buffer solution to 2.5. Sulfonated dextran with a molecular weight of 500,000 was added to the above buffer solution at a ratio of 2.0% (w / v), dissolved and then filtered through a 0.22 μm filter membrane and ultrasonicated for 5 minutes. Dissolve rotigotine and trihexyphenidyl in ultrapure water to obtain stock samples with a concentration of 10 mmol / L, and store them at 4°C. During sample injection, the stock sample was diluted with running buffer to obtain the sample to be separated with a concentration of 0.1 mmol / L.
[0076] 2. Instrument Preparation
[0077] The separation process was carried out in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com