Binary solution type preparation for intravenous injection and intracerebral injection
A binary solution and solvent technology, which can be used in medical preparations with non-active ingredients, medical preparations containing active ingredients, drug delivery, etc., can solve problems such as instability of temozolomide
Inactive Publication Date: 2009-10-21
北京京卫燕康药物研究所有限公司
View PDF2 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
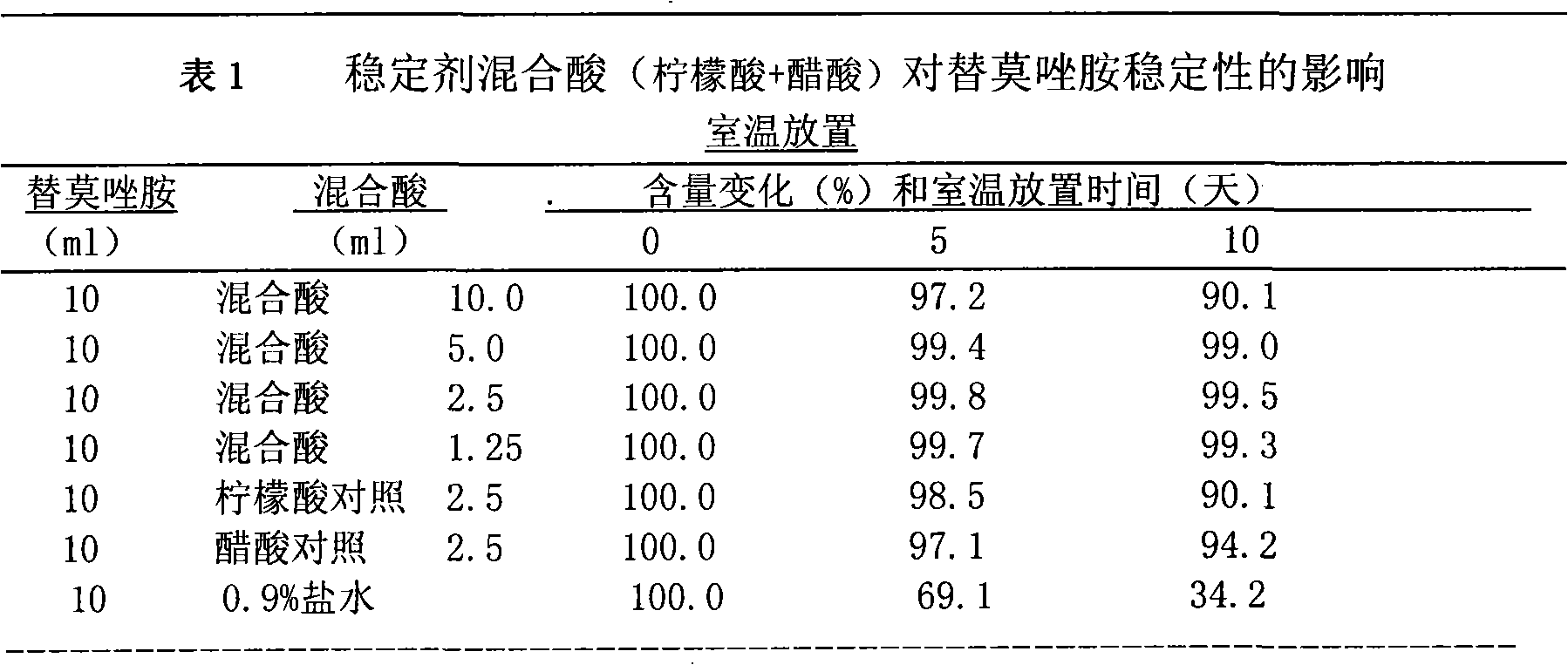
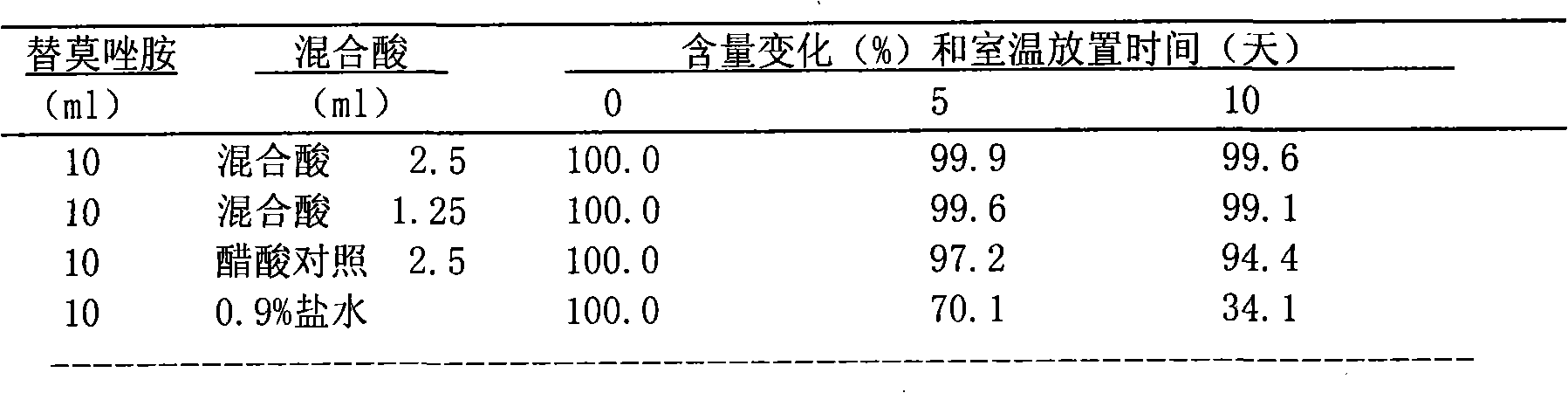
The present invention 1. adopts binary preparation method, promptly medicine and solvent are packed in two different preparation bottles respectively, and solvent is injected in the medicine during use, uses after dissolving, and binary preparation method can solve the long-term stability problem of medicine (more than 2 years ); the present invention ② finds a solvent that stabilizes temozolomide, which solves the problem of instability of temozolomide in the infusion process, and the solvent is non-toxic and non-irritating to the human body, and can be used locally in the brain
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
[0023]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
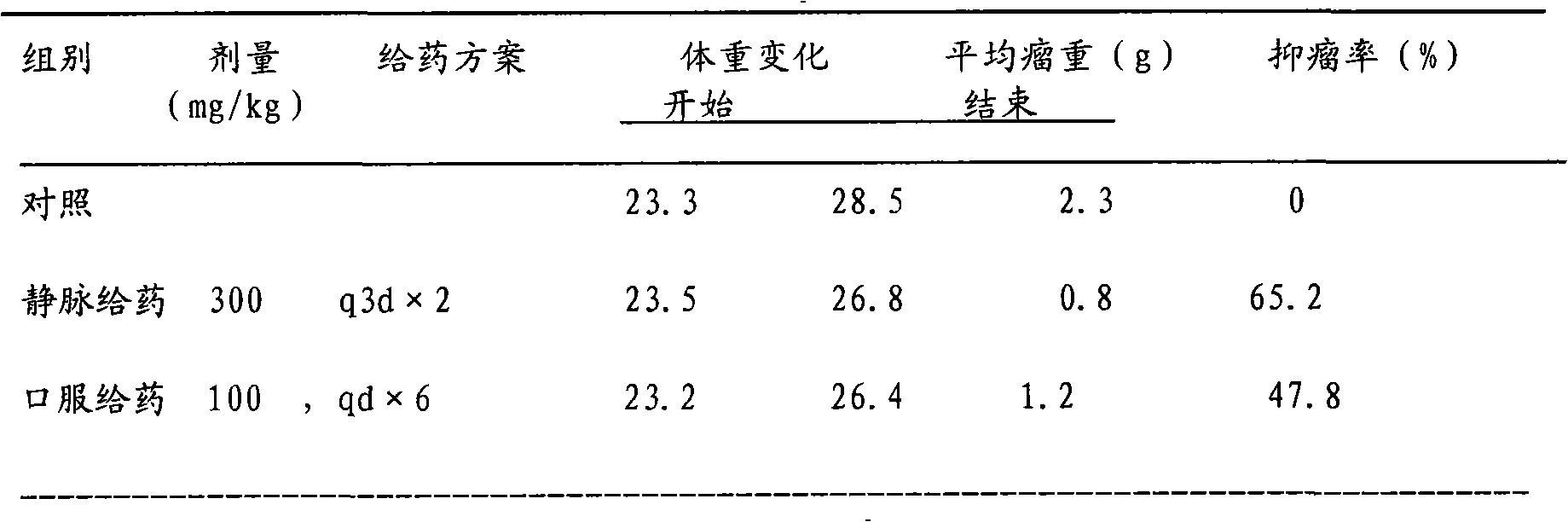
The invention discloses a binary solution type preparation for intravenous injection and intracerebral injection. The main active ingredient of the preparation is temozolomide which is a binary system, and the temozolomide comprises temozolomide aseptic powder and a solvent for dissolving medicines. The combination and the compatibility of the solvent can be used for obtaining a service type temozolomide solution. The invention has the characteristics that the binary solution type preparation is stable at room temperature for more than two years; the prepared medical solution is stable at roomtemperature for at least ten days and can meet the use requirement of injection administration; and the solvent has no toxin or incitation, and the prepared medicine can be used for intracerebral administration in an operation and can be used for intratumor injection local chemotherapy by stereotaxic technique.
Description
Technical field: [0001] The invention relates to a solution type preparation of medicine, in particular to a new preparation composed of Temozolomide and a solvent for dissolving Temozolomide for intravenous and intracerebral local application. technical background: [0002] Temozolomide (Temozolomide) chemical name 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]-1,2,3,5-tetrazine-8-amide, by Schering- Developed by Plow Company. In 1999, the FDA approved its oral capsule formulation for the treatment of adult glioblastoma and melanoma, especially the first-line treatment of glioblastoma multiforme or anaplastic astrocytoma. According to clinical statistics in Europe and the United States, the incidence of primary intracranial malignant tumors is 21 / 100,000, and gliomas account for about 60%, of which highly malignant gliomas account for 70%-85%. The tumor is incompletely differentiated and penetrates deep into the nerve cells, so it is impossible to completely remove it by surger...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/08A61K31/4188A61K47/12A61P35/00
Inventor 张秀国冯志媛尹永祥
Owner 北京京卫燕康药物研究所有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com